Abstract
Motherhood in mammals involves tremendous changes throughout the body and central nervous system, which support attention and nurturing of infants. Maternal care consists of complex behaviors, such as nursing and protection of the offspring, requiring new mothers to become highly sensitive to infant needs. Long-lasting neural plasticity in various regions of the cerebral cortex may enable the perception and recognition of infant cues, important for appropriate caregiving responses. Recent findings have demonstrated that the neuropeptide oxytocin is involved in a number of physiological processes, including parturition and lactation and dynamically shaping neuronal responses to infant stimuli as well. Here, we review experience-dependent changes within the cortex occurring throughout motherhood, focusing on plasticity of the somatosensory and auditory cortex. We outline the role of oxytocin in gating cortical plasticity and discuss potential mechanisms regulating oxytocin release in response to different sensory stimuli.
Similar content being viewed by others
Introduction
Maternal care is critical for child survival and health (Rilling and Young 2014). Early mother-infant relationships have long-term effects on the cognitive, behavioral and emotional development of offspring. New mothers themselves undergo dramatic endocrinological and physiological changes supporting the establishment and maintenance of maternal caregiving, including changes throughout the central nervous system (Bornstein et al. 2017; Bridges 2016; Kim et al. 2016; Olazábal et al. 2013). Healthy maternal sensitivity is characterized by the ability to reliably recognize and respond to infant cues, thus initiating appropriate caregiving responses (Dulac et al. 2014; Insel and Young 2001; Kohl and Dulac 2018; Kurth et al. 2014; Marlin et al. 2015; Parsons et al. 2017; Rickenbacher et al. 2017; Rilling and Young 2014).
Mammalian infants interact with adults in specific ways and their physiological needs are signaled by stereotypic behaviors that share general principles among species (Blass and Teicher 1980; Lingle et al. 2012; Matthiesen et al. 2001; Newman 2007; Zeifman 2011). These signals from the newborn, in turn, trigger complex behaviors in the mother: direct somatic contact with the offspring mainly initiates a nursing response, while other sensory stimuli like cries or odors contribute essentially to arousal and orientation (Bridges 2016; Swain et al. 2011).
Growing evidence indicates that the emergence of different aspects of maternal behavior relies on experience-dependent changes within the maternal brain that enable the processing of sensory cues from the newborn (Bridges 2015; Kim et al. 2016; Kohl and Dulac 2018). Among other changes, long-lasting plasticity in that somatosensory (Rosselet et al. 2006; Xerri et al. 1994) and auditory (Cohen et al. 2011; Cohen and Mizrahi 2015; Liu et al. 2006; Liu and Schreiner 2007; Marlin et al. 2015; Shepard et al. 2016; Tasaka et al. 2018) cortex have recently been shown to contribute to increased responsiveness to infant cues. Most of these studies have been performed in rodents, amenable to techniques for selective monitoring and manipulation of single cells and networks in vivo.
An important question is what mechanisms drive the increase in salience of social information from the offspring. The neuropeptide oxytocin can selectively gate cortical responses to infant stimuli to subsequently enable maternal care (Febo et al. 2005; Marlin et al. 2015). Oxytocin is synthesized in the paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus (Grinevich 2017; Ludwig and Leng 2006) and exerts its central actions via the oxytocin receptor (Ludwig and Leng 2006). Oxytocin is responsible for various prosocial functions in mammals such as parental care, pair bonding and empathy (Feldman 2016; Fineberg and Ross 2017; Insel and Young 2001). Thus, oxytocin priming of cortical circuits in new mothers may allow further experience-dependent reshaping of neural representations of infant cues.
Plasticity in the somatosensory cortex during motherhood
Mammalian life cycle consists of multiple milestones including early postnatal development, reaching sexual maturity and the transition to motherhood. Novel experiences trigger adaptive changes in cortical circuits to allow the perception and the recognition of sensory stimuli with behavioral significance and enable adaptive behaviors (Carcea and Froemke 2013; Froemke 2015). Although the cortical critical period ends relatively early in the postnatal development, cortical networks maintain the ability to undergo plasticity with experience or learning during adulthood (Brecht et al. 2018; Carcea and Froemke 2013; Gilbert and Li 2012; Hensch 2005). Importantly, the neural processing of socially relevant stimuli is supported by experience-dependent changes in cortical circuits (Brecht et al. 2018). Motherhood is a dramatic natural experience that requires the acquisition of complex behaviors related to infant needs. Dynamic changes in neural representations of infant cues in the sensory cortex may provide a substrate to support attention and nurturing towards the young.
In the primary area of the somatosensory cortex (S1), the representation of the ventrum is expanded in primiparous lactating rats compared to virgins (Rosselet et al. 2006; Xerri et al. 1994). This is displayed by a twofold increase of the area representing the nipples and areolae, likely due to strengthened sensory inputs related to nursing (Fig. 1a). Indeed, in postpartum rodents, the frequency and duration of tactile stimulation from suckling pups is dramatically increased as infants spend around 50% of their awake time nursing (Champagne et al. 2007; Grota and Ader 1974). Stronger afferent inputs from the nipples, resulting from the continuous nursing, may induce activity-dependent plasticity in S1 neurons similarly to the expansion of the female genital cortex during puberty (Lenschow et al. 2017, 2016).
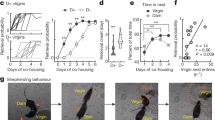
Cortical plasticity during motherhood. Somatosensory map representing the ventrum in S1 of virgins (a), non-lactating (a′) and lactating (a″) dams at 16 days postpartum. Note the increase in map area (mm2) in lactating dams. Adapted with permission from Xerri et al. (1994). (b) PSTH of neural responses from dams and virgins to pup call playback (horizontal bar) from three recording sites in A1 having different characteristic frequencies (0–20, 20–40 and 40–80 kHz); scale 50 spikes/s (vertical bar). Note the earlier onset and higher amplitude of the responses in dams. Adapted with permission from Liu and Schreiner (2007). Whole-cell recordings in A1 of dams (c) and virgins (c′). Spiking (c″) and synaptic (c′″) responses to pup calls playback. Note the temporal precision of spiking responses and the correlation of synaptic excitation and inhibition (rei-best) in dams (c″″, d) compared to virgins (c′″″, d′). Adapted with permission from Marlin et al. (2015)
Changes in the S1 representational territory are strongly related to nursing experience as it is absent in postpartum rats (Fig. 1a, “Non-lactating”) whose litters have been removed on the day of birth (Xerri et al. 1994). This implies that active nursing is required for S1 map plasticity, rather than parturition alone. In addition, increase in dendritic spine density in layer III and V pyramidal neurons of the somatosensory cortex is more prominent in lactating dams after 2 weeks of lactation onset, compared to pregnant females (Chen et al. 2017) but the thickness of the rat somatosensory cortex is increased almost immediately after parturition (Hamilton et al. 1977).
Experience-dependent changes in cortical responses continue throughout the post-partum period. Receptive fields of S1 neurons representing the nipple-bearing skin of the ventrum in lactating rats are reduced prior to S1 map extension, at 1 week after nursing onset (Rosselet et al. 2006). This refinement of cellular responses allows for finer topographic representation. However, the size of receptive fields return to baseline levels at 3 weeks after nursing onset, prior to weaning. In contrast, map extension appears much later (at 2 weeks after nursing onset) but it is maintained until weaning (Rosselet et al. 2006). In addition, increased dendritic spine density in layer III and V pyramidal neurons remains up to 6 weeks after weaning (Chen et al. 2017).
Nursing-induced cortical plasticity promotes strong activation of S1 in response to nipple stimulation, which is not restricted to natural nursing behavior. Indeed, increased activation of S1 during suckling can also be mimicked by artificial suction or nipple rubbing, suggesting that adaptive changes in the cortex of lactating dams are quite stimulus-specific and do not necessarily require the presence of the pup itself to engage these mechanisms (Febo et al. 2008).
Plasticity in the auditory cortex during motherhood
Neural representations of pup vocalizations in the auditory cortex also undergo experience-dependent plasticity with the transition to motherhood (Fig. 1b–d), thus enhancing auditory responses to pup calls (Cohen et al. 2011; Cohen and Mizrahi 2015; Liu et al. 2006; Liu and Schreiner 2007; Marlin et al. 2015; Shepard et al. 2016; Tasaka et al. 2018). Pup distress calls are overrepresented in dams compared to virgins, leading to a stronger activation of the deep layers and increased noise correlation in layer II/III of the auditory cortex in lactating females (Cohen et al. 2011; Rothschild et al. 2013). In addition, pup distress calls reliably drive individual neurons in mouse primary auditory cortex (A1) in a temporally precise manner (Marlin et al. 2015). These changes are paralleled by a shift in the balance between excitation and inhibition (Cohen and Mizrahi 2015; Marlin et al. 2015) and a greater number of pup call-responding neurons (Tasaka et al. 2018). Furthermore, the overall tonotopic organization of A1 is not modified by motherhood compared to the S1 representational territory of the ventrum (Rosselet et al. 2006; Shepard et al. 2016, 2015; Xerri et al. 1994), perhaps because neurons responding to pup calls are fairly sparse and found throughout the entirety of A1 irrespective of frequency.
Importantly, auditory cortical plasticity appears to endure longer than representational changes to S1. As mentioned above, somatosensory map extension and receptive field shifts are observed exclusively during the lactation period (during the 3 weeks after parturition); during this period, dams are engaged in active nursing and receive continuous tactile stimulation from the suckling pups (Rosselet et al. 2006; Xerri et al. 1994). In contrast, changes in the auditory cortex that might enhance the detection of infant vocalizations have been shown to persist up to several weeks after weaning when pups do no longer need extensive maternal care. Indeed, increase in neuronal responsiveness to the bout structure of pup distress calls can be observed up to a week after weaning (Liu et al. 2006). Improved pup call detection and discrimination information also persists after weaning as decreased latency of response onset and increased magnitude of auditory cortex responses to pup calls (Fig. 1b) are observed in weaned dams (Liu and Schreiner 2007), although a recent study reported that single-cell responses to pup calls in dams disappear at 10 days post-weaning (Tasaka et al. 2018). In the auditory cortex of lactating, as well as weaned (up to 3 weeks) dams, an increase in the neuronal responses to pup calls in ultrasound-responding regions, while a decrease in responses in areas that respond to low-frequency sounds are observed (Shepard et al. 2016). This is paralleled by a decrease in the spontaneous activity of single neurons in weaned dams (Galindo-Leon et al. 2009; Lin et al. 2013). Such a contrast enhancement may further strengthen the recognition of natural pup sounds with behavioral relevance in dams. In any case, behavioral responses of experienced mothers persist throughout life to pup distress calls. Studies from human mothers show that reactivity to infant auditory cues builds over time. Vocalization processing of baby cries is increased in more experienced mothers (Bornstein et al. 2017; Parsons et al. 2017), which possibly improves the recognition of the behavioral meaning of these sounds and promotes appropriate caregiving responses (Kurth et al. 2014).
The behavioral and broader evolutionary advantage of these long-lasting changes in the auditory cortex in response to infant vocalizations remains unexplored. A possible interpretation can be that such plasticity of auditory responses may lead to increased sensitivity of a broader range of social vocalizations (including infant and adult calls), which can promote robust parental and pro-social behaviors. This, in turn, will have a positive outcome for future maternal or social experience and therefore be crucial for the survival of different mammalian species.
Potential roles of oxytocin for cortical plasticity
What neurobiological mechanisms might be recruited for changes to cortical networks that might help guide behavior? In many cases, long-lasting plasticity in the adult cortex requires activation of subcortical modulatory systems that provide behavioral context to incoming sensory signals (7Froemke 2015). In the case of maternal behavior, oxytocin is a main candidate for hormonal regulation and neural circuit modulation relevant for parenting. There are two complementary circuit mechanisms that might be engaged. First, increased sensory inputs related to infant needs might efficiently drive oxytocin neurons (directly or indirectly). Second, enhanced oxytocin release might promote cortical plasticity to further increase the detection of infant stimuli. In the first case, experience-dependent plasticity of hypothalamic oxytocin neurons might increase their activity in response to pup stimuli (touch, sound) and promote faster and more reliable peripheral and central release of oxytocin. In the second case, central oxytocin release from the hypothalamus may also provide a feedforward modulation of cortical activity.
Projections arising from sensory or higher-order cortical areas might provide an important input to PVN oxytocin neurons, leading to increased oxytocin release in downstream areas, possibly including the cerebral cortex and/or upregulating oxytocin synthesis in the hypothalamus. Indeed, the latency of the milk ejection reflex in lactating rats decreases at longer postpartum times (Jans and Woodside 1987) and the sound of a baby cry activates the hypothalamus (Lorberbaum et al. 2002) and triggers oxytocin plasma elevations in human mothers (McNeilly et al. 1983). This suggests an activity-dependent potentiation of oxytocin neuronal responses to infant cues leading to increased activation of the hypothalamus following different sensory stimuli.
Interestingly, oxytocin levels in the barrel field of S1 have been shown to vary with sensory experience, with higher oxytocin after prolonged sensory stimulation and lower oxytocin following sensory deprivation. Increased sensory experience through environmental enrichment results in higher oxytocin mRNA levels in the hypothalamus and oxytocin peptide levels in S1 (Zheng et al. 2014). Conversely, hypothalamic oxytocin mRNA levels and the number of oxytocin neurons in PVN (but not in SON) are reduced after sensory deprivation from birth through both dark-rearing and whisker deprivation (Zheng et al. 2014). This implies that oxytocin neurons are particularly sensitive to variations in sensory inputs and that oxytocin release from the PVN of dams may be influenced by the duration and the intensity of sensory stimulation from the pups.
Neuromodulation of cortical activity by oxytocin is important for female reproductive and maternal behavior (Marlin et al. 2015; Nakajima et al. 2014; Sabihi et al. 2014). Oxytocin in A1 and medial prefrontal cortex (mPFC) mediates pup retrieval (Marlin et al. 2015; Sabihi et al. 2014). Blockade of mPFC oxytocin receptors enhances maternal aggression (Sabihi et al. 2014) and central oxytocin administration decreases the activation of the prelimbic mPFC when dams are presented with predator odor, likely decreasing fear response (Febo et al. 2009). Oxytocin is also involved in female sexual behavior during estrous by modulating the activity of interneurons in the prefrontal cortex (Nakajima et al. 2014). Both S1 and A1 are enriched in oxytocin receptors, with additional lateralization of A1 oxytocin receptor expression such that more cells express these receptors in female left A1 than right A1 (Marlin et al. 2015; Mitre et al. 2016). Interestingly, the left auditory cortex is responsible for neural processing of cry perception in humans (Montoya et al. 2012; Sander and Scheich 2005). Variations in the human oxytocin receptor gene are associated with different sensitivity to infant crying (Riem et al. 2011) and different degrees of parental responsiveness measured by mother-infant interactions (Michalska et al. 2014). Oxytocin receptors in A1 are mainly expressed by parvalbumin and somatostatin interneurons (Marlin et al. 2015; Mitre et al. 2016). Activation of these receptors via oxytocin application decreases evoked and spontaneous inhibitory transmission and increases spiking output in A1 (Marlin et al. 2015; Mitre et al. 2016). Oxytocin leads to a similar increase in the temporal precision of spike output in the hippocampus, although it acts by different mechanisms. Activation of the oxytocin receptor in the hippocampus results in increased inhibitory tone by increasing the activity of fast-spiking interneurons and a subsequent decrease in the background firing of pyramidal neurons (Owen et al. 2013). Oxytocin enables A1 plasticity in vivo, thus enhancing responses to different acoustic stimuli (infant vocalizations and pure tones) in virgins and speeds the acquisition of maternal behavior (Marlin et al. 2015; Mitre et al. 2016). Endogenous oxytocin release by optogenetic stimulation of PVN oxytocin neurons (or oxytocin fibers in A1) or exogenous oxytocin application, when paired with pup distress calls, enables rapid rescaling of excitatory and inhibitory synaptic transmission. This excitatory-inhibitory balance results in highly reliable, temporally precise activation of cortical neurons in dams and experienced virgins (Marlin et al. 2015). Therefore, central oxytocin release may increase the salience of incoming sensory inputs in particular contexts and promote activity-dependent plasticity of cortical networks. Oxytocin also seems to be involved in the degree of S1 activation during nursing episodes (Febo et al. 2005) but its role in S1 plasticity and its timecourse related to cortical receptive fields and map reshaping in dams remains unknown.
Central actions of oxytocin are mediated by oxytocin receptors on cortical interneurons but the exact nature of cortical oxytocin remains somewhat debated (Marlin et al. 2015; Mitre et al. 2016; Stoop 2012; Zheng et al. 2014). Blockade of oxytocin signaling by intraventricular infusion of the oxytocin antagonist OTA decreases fMRI signals in the somatosensory cortex of dams during nursing (Febo et al. 2005). However, Zheng et al. (2014) did not identify any direct projections from the PVN to a sensory cortex using retrograde tracing. In contrast, direct inputs from PVN to A1 were demonstrated, as anterograde tracing of PVN oxytocin projections showed the presence of axonal fibers in both left and right A1 (Marlin et al. 2015). Importantly, oxytocin fibers in A1 are functional, as their optogenetic stimulation results in similar effects as direct oxytocin application: reduction of synaptic inhibition and increased firing of cortical neurons in vivo (Marlin et al. 2015; Mitre et al. 2016). Functional oxytocinergic axons have also been found in various central areas such as the lateral part of the central amygdala (Knobloch et al. 2012), piriform cortex (Mitre et al. 2016), anterior olfactory nucleus (Oettl et al. 2016) and ventral tegmental area (Hung et al. 2017).
Spatiotemporal scales of cortical oxytocin signaling
Somatic stimulation from the periphery triggers oxytocin release in the blood during parturition and suckling (Fig. 2). Oxytocin neurons are activated in response to the stretch of the cervix to further induce peripheral oxytocin release that stimulates uterine contractions, required for the expulsion of the fetus during labor (Chan and Chen 1992; Matthiesen et al. 2001; Summerlee 1981). Similarly, massaging and licking of the nipples from the young during suckling triggers oxytocin release important for the contraction of the myoepithelial cells in the breast and subsequent milk ejection (Brown and Moos 1997; Ellendorff et al. 1982; Freund-Mercier et al. 1988; Nishimori et al. 1996; Paisley and Summerlee 1984). Somatosensory information from the uterus and the nipples enters the dorsal horn of the spinal cord through sacral and thoracic C-fibers of the spinal nerve via the dorsal root ganglion and reaches the brain through the lateral spinothalamic tract or the spinocervicothalamic tract; it is then relayed through the ventral posterolateral thalamic nucleus to S1 (Dubois-Dauphin et al. 1985a, b; Eayrs and Baddeley 1956; Fukuoka et al. 1984). Oxytocin neurons in the PVN and SON receive somatosensory information through A2 adrenergic fibers originating from the nucleus tractus solitarius in the brainstem (Cunningham and Sawchenko 1988; Douglas et al. 2001; Meddle et al. 2000; Onaka et al. 1995; Raby and Renaud 1989). Norepinephrine acts on local glutamatergic circuits within the hypothalamus to activate oxytocin neurons (Boudaba et al. 2003; Daftary et al. 1998; Randle and Bourque 1986; Yamashita et al. 1987). More recently, projections from PVN to noradrenergic neurons in NTS have also been described (Geerling et al. 2006). Oxytocin is released from the posterior pituitary into the bloodstream to induce smooth muscle contraction of the uterus during parturition, or the milk ducts during suckling (Fig. 2).
Peripheral release of oxytocin during parturition and suckling. Sensory information from the uterus (dilation of the cervix) during parturition and the nipples during suckling is carried by c-type sensory fibers via the dorsal root ganglion (DRG) in the spinal cord where they send ascending projections via the spinothalamic or the spinocervicothalamic tract, to the ventral posterolateral nucleus of the thalamus (VPL). VPL then projects to the somatosensory cortex (S1). Projections from S1 to the PVN have not been demonstrated. A2 adrenergic fibers from the nucleus tractus solitarius (NTS) release noradrenaline (NE) in PVN and SON in response to suckling and during parturition and contribute to the burst firing of oxytocin (OT) neurons and subsequent oxytocin release in the blood. Peripheral oxytocin induces contractions of the uterus and the milk ducts during the milk ejection reflex (MER). PVN also projects to the auditory cortex (A1)
Oxytocin plasma levels peak for a few minutes immediately preceding the expulsive phase of labor (Dawood et al. 1983; Gilbert et al. 1994; O’Byrne et al. 1986; Thornton et al. 1988) and remain elevated for up to 45 min postpartum in humans (Nissen et al. 1995). However, it is unknown whether oxytocin is simultaneously released in central brain regions during that time, or whether this postpartum oxytocin spike is required for cortical plasticity and increased sensitivity towards infant cues. One study in human subjects showed that vaginal delivery has a positive effect on the baby cry response in the auditory cortex of human mothers (Swain et al. 2008).
During nursing, plasma oxytocin levels peak at a slower timescale in the order of several minutes (Dawood et al. 1981; Lucas et al. 1980; Weitzman et al. 1980) and follow a pulsatile pattern (Freund-Mercier et al. 1988; Jonas et al. 2009). In human mothers, periods of massage-like hand movements performed by the baby prior to suckling are followed by an increase in maternal oxytocin plasma levels lasting for several minutes (Matthiesen et al. 2001). This suggests that not only the suckling itself but also skin-to-skin contact with the child are able to induce high oxytocin in mothers. Thus, extensive nursing and contact with the infant would trigger pulsatile release of oxytocin associated with individual episodes of the milk ejection reflex but altogether leading to an increased oxytocinergic tone in the PVN (Bains 2002; Ludwig and Leng 2006).
This positive feedback loop of oxytocin-induced-oxytocin release within the PVN, induced by nursing, may contribute to a parallel oxytocin release in central areas like the auditory cortex. Breastfeeding has been shown to result in increased activation of various cortical regions in response to mother’s own baby’s cry (Kim et al. 2011) and promotes maternal sensitivity towards infant distress (Edwards et al. 2015; Pearson et al. 2011). Suckling itself results in activation of various cortical areas (Febo et al. 2008, 2005; Ferris et al. 2005), which further suggests that high levels of central oxytocin, induced by nursing experience, may drive cortical plasticity. Importantly, oxytocin plasma levels are decreased in mothers with postpartum depression who also present breastfeeding difficulties (Brummelte and Galea 2016; Kim et al. 2014). Depressed mothers have decreased sensitivity to infant distress vocalizations and are less likely to initiate adequate caregiving responses (Esposito et al. 2017; Murray et al. 1996).
Actions of central oxytocin are fast, on the order of seconds to minutes (Dölen et al. 2013; Hung et al. 2017; Knobloch et al. 2012; Mitre et al. 2016; Ninan 2011; Oettl et al. 2016; Owen et al. 2013; Tang et al. 2014; Xiao et al. 2018; Xiao et al. 2017). Oxytocin release enables the detection of infant vocalizations in A1 in vivo and drives quick and efficient maternal responses (Marlin et al. 2015). Moreover, oxytocin may also have long-lasting actions in cortical areas, as acute oxytocin injection in S1 results in increased excitatory transmission, measured in brain slices at 1 day after treatment (Zheng et al. 2014). Therefore, it is possible that map expansion and receptive field plasticity (Rosselet et al. 2006; Xerri et al. 1994) may be due to an increased input from sensory afferents, together with higher oxytocin in S1.
Contraction of the uterus during parturition, as well as contractions of the milk ducts allowing for the milk letdown during suckling, are triggered by a well-characterized rhythmic pattern of oxytocin neuronal firing (Fig. 3). Oxytocin neurons in PVN and SON exhibit intermittent high-frequency bursts consisting of a 50–100 Hz firing rate within bursts and lasting for 1–2 s (Brown and Moos 1997; Paisley and Summerlee 1984; Summerlee 1981). Rhythmic bursting activity in oxytocin neurons is generated by afferent excitatory inputs (Jourdain et al. 1998). Each burst occurs every 5–10 min and it is correlated with the rise of oxytocin plasma levels that occurs within a few seconds after neuronal bursting (Dawood et al. 1981; Lincoln and Wakerley 1975). These bursts are followed with a period of inactivity during several seconds. Therefore, parturition and suckling trigger a stereotypic pattern of rhythmic activity in oxytocin neurons and intermittent peripheral oxytocin release. In addition, during suckling, there is a linear increase in their firing rates following increased stimulation (i.e., number of pups that suckle) (Lincoln and Wakerley 1975).
Burst-firing in oxytocin neurons. a Firing rate of one PVN neuron recorded with an extracellular electrode in a freely moving rat during parturition. Delivery of fetus (D; black triangles) or placenta (white triangles). Abdominal straining movements (arrow). Note the increase in firing rate preceding each delivery. Adapted with permission from Summerlee (1981). b Firing rate of two SON neurons during paired extracellular recordings in anesthetized rat during suckling. Dashed line depicts the onset of suckling. Bursts are marked by an asterisk. Note the synchronous bursting between both cells. Adapted with permission from Brown and Moos (1997). c Extracellular recording (top, integration; bottom, unitary responses) of one SON neuron in anesthetized rat during suckling. The peak in intramammary pressure corresponds to a pulse of about 0.5–1.0 m.u. oxytocin. Neurosecretory response (NE); milk ejection (ME). Note the delay between increased neuronal activity and the peak in intramammary pressure. Adapted with permission from Lincoln and Wakerley (1975)
However, aside from parturition and suckling, it is unclear when oxytocin neurons are activated by other infant-related stimuli such as touch (e.g., pup grooming in rodents, skin-to skin contact in humans) or sound (e.g., infant vocalizations), or whether these patterns of activity are distinct from the well-known high-frequency burst firing during milk ejection reflex (Lincoln and Wakerley 1975). It is possible that other sensory stimuli that—unlike suckling—do not occur in a rhythmic way (Ellendorff et al. 1982; German et al. 1997; Westneat and Hall 1992), would result in more continuous firing patterns, enabling sustained oxytocin release in central areas.
Importantly, oxytocin neurons receive various inputs (Brown et al. 2013; Johnson et al. 2018), which may be activated in different contexts and, thus, promote or inhibit the stereotyped bursting activity. Burst generation and the milk ejection reflex are occluded by low-frequency activation of the lateral and medial septum (Boudaba and Poulain 1991; Lebrun et al. 1983; Lebrun and Poulain 1982), which plays a role in maternal aggression important for offspring protection (Lonstein and Gammie 2002). Hyperosmotic stimulation also results in interruption of bursting activity and inhibition of the milk ejection reflex, while GABAergic inputs have been shown to facilitate burst firing in oxytocin neurons (Moos 1995). In contrast, simulations shows that when excitation and inhibition are balanced, oxytocin neurons exhibit a linear response to proportionate changes in input rate (Leng et al. 2001). Therefore, oxytocin neurons display two contrasting patterns of activity: bursting and continuous firing, which may be triggered by the recruitment of different inputs. One possibility is that various distal auditory and/or olfactory infant cues that induce appetitive (pup retrieval) and consummatory (pup licking) behaviors in dams (Numan 2007) may result in a tonic firing of oxytocin neurons. In contrast, burst firing may occur exclusively during parturition to induce contractions of the uterus and expulsion of the fetus and during nursing to allow the milk letdown. Interestingly, it has been proposed that increased arousal would block the milk ejection reflex and therefore occludes rhythmic bursting in oxytocin neurons in rats and rabbits (Lincoln et al. 1980; Summerlee and Paisley 1982) but not in pigs (Poulain et al. 1981). Increased arousal occurs during states of high maternal motivation, when hearing the calls of distressed pups, for example (Newman 2007; Numan 2007). Some aspects of maternal behavior, such as pup approach and retrieval in response to pup distress calls, as well as pup licking, requires dopamine signaling, while nursing requires inhibition of dopamine (Hansen et al. 1991a, b; Johns et al. 2005; Keer and Stern 1999; Silva et al. 2001; Stern and Taylor 1991; Tay et al. 1993). Importantly, this suggests that high maternal arousal has an antagonistic action on nursing but not on central oxytocin release, as pup retrieval is dependent on oxytocin signaling in A1 (Marlin et al. 2015). Therefore, different sensory stimuli (distal such as vocalizations, or proximal such as suckling) might induce contrasting activity patterns in oxytocin neurons resulting in either peripheral or central oxytocin release.
Responses to pup distress calls in A1 of dams can be mimicked by pairing calls with optogenetic activation of PVN oxytocin axons in A1 of virgins (Marlin et al. 2015). This increase in oxytocin signaling in the cortex of dams may be driven by the release of oxytocin from the PVN in response to infant vocalizations. However, it is unclear what inputs to PVN oxytocin neurons might provide this information and neuronal drive (Brown et al. 2013; Numan and Young 2016). Although it still remains unexplored which inputs to PVN and what firing patterns of oxytocin neurons are sufficient to trigger oxytocin release in central targets (Chini et al. 2017), there is substantial evidence that they may be different than burst-firing. While rhythmic bursting activity, associated with the milk ejection reflex and peripheral oxytocin release from the pituitary, consists of intraburst frequencies of 50–100 Hz (Brown and Moos 1997; Paisley and Summerlee 1984; Summerlee 1981), optogenetic stimulation of either PVN oxytocin neurons or their projecting fibers in various central and peripheral regions, have demonstrated that lower frequencies of continuous firing are sufficient to trigger oxytocin release. Optogenetic stimulation of oxytocin neurons in the PVN at 30 Hz promotes social learning (Choe et al. 2015). Optogenetic stimulation of PVN oxytocinergic inputs in the lateral part of the central amygdala at 30 Hz for 20 s increases the firing rate of amygdala neurons in brain slices and attenuates freezing behavior in animals exposed to fear context in vivo (Knobloch et al. 2012). The same pattern of optogenetic stimulation applied to PVN oxytocin fibers in SON increases the activity of SON neurons ex vivo and significantly elevates plasma oxytocin levels (Eliava et al. 2016). In addition, PVN oxytocin fibers in the spinal cord, which regulate the activity of the C-fibers, can also be activated by this optogenetic stimulation to modulate inflammatory pain processing (Eliava et al. 2016). Finally, stimulating PVN oxytocin inputs in the anterior olfactory nucleus results in increased excitability and excitatory drive ex vivo (Oettl et al. 2016) and oxytocin release from PVN fibers in the ventral tegmental area promotes sociability (Hung et al. 2017). Repeated optogenetic stimulation of PVN oxytocin axons in A1 at 30 Hz for 1 s, paired with the presentation of pup distress calls in virgins, is sufficient to modify the excitatory-inhibitory balance in vivo and results in temporally precise activation of cortical neurons in response to the calls (Marlin et al. 2015). The same pattern of stimulation in A1 or PVN also has a strong behavioral effect since it significantly decreases the pup retrieval onset in cohoused virgins (Marlin et al. 2015). Importantly, optogenetic oxytocin release from axons can be induced at a much lower stimulation frequency at an increased duration of 5 Hz during 3 min in different brain structures ex vivo as A1, piriform cortex and PVN itself, resulting in a decrease of inhibitory transmission and increased spiking output (Mitre et al. 2016). Although optogenetic stimulation might produce artificially-synchronous activity across oxytocin neurons or oxytocin axons, these data suggest that oxytocin release in central areas may be triggered by patterns of firing that differ from the well-known bursting activity important for parturition and lactation.
Conclusion
Oxytocin is involved not only in homeostatic regulation of reflexes such as parturition and lactation but it is a crucial part of other aspects of motherhood such as care and protection of the offspring (Marlin et al. 2015; Nakajima et al. 2014; Sabihi et al. 2014). Oxytocin mediates a broad spectrum of prosocial behaviors such as empathy, pair bonding, mating and social reward (Dölen et al. 2013; Hung et al. 2017; Insel and Young 2001; Nakajima et al. 2014; Rogers-Carter et al. 2018). Therefore, studying oxytocin circuits controlling maternal behavior will advance our understanding of the mammalian social behavior network.
Oxytocin gates the representation of infant vocalizations in A1 (Marlin et al. 2015) but it remains unknown whether it is also important for the onset and/or maintenance of plasticity in S1 (Rosselet et al. 2006; Xerri et al. 1994). The oxytocin receptor is expressed in several cortical areas, including A1, S1, mPFC, insular cortex, entorhinal cortex and piriform cortex (Knobloch et al. 2012; Mitre et al. 2016; Rogers-Carter et al. 2018; Sabihi et al. 2014). However, it remains unexplored how maternal oxytocin might control these areas.
New mothers can recognize the different needs of the young and appropriately respond to them. Infant stimuli trigger simultaneous responses in different brain areas of mothers (Febo et al. 2008; Lorberbaum et al. 2002; Sander and Scheich 2005) but it remains to be explored if oxytocin acts simultaneously in different cortical areas to gate these responses, or, alternatively, if oxytocin signaling is restricted to specific regions. Finally, the transition to motherhood may trigger long-lasting changes in oxytocin signaling in central areas, which have additional consequences for future social experience or cognitive processes. Oxytocin signaling in the insular cortex differentially regulates affection towards juveniles or adults (Rogers-Carter et al. 2018). An important future research direction would be to explore how cortical oxytocin during motherhood might affect the perception of infant and adult social cues.
References
Bains JS (2002) Dendritic action potentials in magnocellular neurons. Prog Brain Res 139:225–234. https://doi.org/10.1016/S0079-6123(02)39019-8
Blass EM, Teicher MH (1980) Suckling. Science 210(4465):15–22. https://www.ncbi.nlm.nih.gov/pubmed/6997992. Accessed 11 May 2018
Bornstein MH, Putnick DL, Rigo P, Esposito G, Swain JE, Suwalsky JTD, Su X, Du X, Zhang K, Cote LR, De Pisapia N, Venuti P (2017) Neurobiology of culturally common maternal responses to infant cry. Proc Natl Acad Sci 114:E9465–E9473. https://doi.org/10.1073/pnas.1712022114
Boudaba C, Poulain DA (1991) Further evidence that the septum is not part of the main pathway of the milk ejection reflex in the rat. J Neuroendocrinol 3:199–204. https://doi.org/10.1111/j.1365-2826.1991.tb00263.x
Boudaba C, Di S, Tasker JG (2003) Presynaptic noradrenergic regulation of glutamate inputs to hypothalamic magnocellular neurones. J Neuroendocrinol 15:803–810. https://doi.org/10.1046/j.1365-2826.2003.01063.x
Brecht M, Lenschow C, Rao RP (2018) Socio-sexual processing in cortical circuits. Curr Opin Neurobiol 52:1–9. https://doi.org/10.1016/j.conb.2018.04.003
Bridges RS (2015) Neuroendocrine regulation of maternal behavior. Front Neuroendocrinol 36:178–196. https://doi.org/10.1016/j.yfrne.2014.11.007
Bridges RS (2016) Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Horm Behav 77:193–203. https://doi.org/10.1016/j.yhbeh.2015.09.001
Brown D, Moos F (1997) Onset of bursting in oxytocin cells in suckled rats. J Physiol 503:625–634. https://doi.org/10.1111/j.1469-7793.1997.625bg.x
Brown CH, Bains JS, Ludwig M, Stern JE (2013) Physiological regulation of magnocellular neurosecretory cell activity: integration of intrinsic, local and afferent mechanisms neuroendocrinology. J Neuroendocrinol 25:678–710. https://doi.org/10.1111/jne.12051
Brummelte S, Galea LAM (2016) Postpartum depression: etiology, treatment and consequences for maternal care. Horm Behav 77:153–166. https://doi.org/10.1016/j.yhbeh.2015.08.008
Carcea I, Froemke RC (2013) Cortical plasticity, excitatoryinhibitory balance, and sensory perception. Prog Brain Res 207:65–90. https://doi.org/10.1016/B978-0-444-63327-9.00003-5. https://www.ncbi.nlm.nih.gov/pubmed/24309251. Accessed 11 May 2018
Champagne FA, Curley JP, Keverne EB, Bateson PPG (2007) Natural variations in postpartum maternal care in inbred and outbred mice. Physiol Behav 91:325–334. https://doi.org/10.1016/j.physbeh.2007.03.014
Chan WY, Chen D-L (1992) Myometrial oxytocin receptors and prostaglandin in the parturition process in the Rat1. Biol Reprod 46:58–64. https://doi.org/10.1095/biolreprod46.1.58
Chen J-R, Lim SH, Chung S-C, Lee Y-F, Wang Y-J, Tseng G-F, Wang T-J, Hong Lim S, Chung S-C, Lee Y-F, Wang Y-J, Tseng G-F, Wang T-J (2017) Reproductive experience modified dendritic spines on cortical pyramidal neurons to enhance sensory perception and spatial learning in rats. Exp Anim 66:61–74. https://doi.org/10.1538/expanim.16-0061
Chini B, Verhage M, Grinevich V (2017) The action radius of oxytocin release in the mammalian CNS: from single vesicles to behavior. Trends Pharmacol Sci 38(11):982–991. https://doi.org/10.1016/j.tips.2017.08.005. https://www.ncbi.nlm.nih.gov/pubmed/28899620. Accessed 11 May 2018
Choe HK, Reed MD, Benavidez N, Montgomery D, Soares N, Yim YS, Choi GB (2015) Oxytocin mediates entrainment of sensory stimuli to social cues of opposing valence. Neuron 87:152–163. https://doi.org/10.1016/j.neuron.2015.06.022
Cohen L, Mizrahi A (2015) Plasticity during motherhood: changes in excitatory and inhibitory layer 2 / 3 neurons in auditory cortex. J Neurosci 35:1806–1815
Cohen L, Rothschild G, Mizrahi A (2011) Multisensory integration of natural odors and sounds in the auditory cortex. Neuron 72:357–369
Cunningham ET, Sawchenko PE (1988) Anatomical specificity of noradrenergic inputs to the paraventricular and supraoptic nuclei of the rat hypothalamus. J Comp Neurol 76:60–76
Daftary SS, Boudaba C, Szabó K, Tasker JG (1998) Noradrenergic excitation of magnocellular neurons in the rat hypothalamic paraventricular nucleus via intranuclear glutamatergic circuits. J Neurosci 18:10619–10628
Dawood MY, Khan-Dawood FS, Wahi RS, Fuchs F (1981) Oxytocin release and plasma anterior pituitary and gonadal hormones in women during lactation. J Clin Endocrinol Metab 52:678–683. https://doi.org/10.1210/jcem-52-4-678
Dawood MY, Med M, Khan-Dawood FS, Ayromlooi J, Tobias M (1983) Maternal and fetal plasma oxytocin levels during pregnancy and parturition in the sheep. Am J Obstet Gynecol 147:584–588. https://doi.org/10.1016/0002-9378(83)90022-4
Dölen G, Darvishzadeh A, Huang KW, Malenka RC (2013) Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 501:179–184. https://doi.org/10.1038/nature12518
Douglas A, Scullion S, Antonijevic I, Brown D, Russell J, Leng G (2001) Uterine contractile activity stimulates supraoptic neurons in term pregnant rats via a noradrenergic pathway. Endocrinology 142:633–644
Dubois-Dauphin M, Armstrong WE, Tribollet E, Dreifuss JJ (1985a) Somatosensory systems and the milk-ejection reflex in the rat. I. Lesions of the mesencephalic lateral tegmentum disrupt the reflex and damage mesencephalic somatosensory connections. Neuroscience 15:1111–1129. https://doi.org/10.1016/0306-4522(85)90256-8
Dubois-Dauphin M, Armstrong WE, Tribollet E, Dreifuss JJ (1985b) Somatosensory systems and the milk-ejection reflex in the rat. II. The effects of lesions in the ventroposterior thalamic complex, dorsal columns and lateral cervical nucleus-dorsolateral funiculus. Neuroscience 15:1131–1140. https://doi.org/10.1016/0306-4522(85)90257-X
Dulac C, O’Connell L, Wu Z (2014) Neural control of maternal and paternal behaviors. Science 345(80):1063–1069
Eayrs JT, Baddeley RM (1956) Neural pathways in lactation. J Anat 90:161–171
Edwards RC, Thullen MJ, Henson LG, Lee H, Hans SL (2015) The association of breastfeeding initiation with sensitivity, cognitive stimulation, and efficacy among young mothers: a propensity score matching approach. Breastfeed Med 10:13–19. https://doi.org/10.1089/bfm.2014.0123
Eliava M, Melchior M, Knobloch-Bollmann HS, Wahis J, da Silva Gouveia M, Tang Y, Ciobanu AC, Triana del Rio R, Roth LC, Althammer F, Chavant V, Goumon Y, Gruber T, Petit-Demoulière N, Busnelli M, Chini B, Tan LL, Mitre M, Froemke RC, Chao MV, Giese G, Sprengel R, Kuner R, Poisbeau P, Seeburg PH, Stoop R, Charlet A, Grinevich V (2016) A new population of parvocellular oxytocin neurons controlling magnocellular neuron activity and inflammatory pain processing. Neuron 89:1291–1304. https://doi.org/10.1016/j.neuron.2016.01.041
Ellendorff BF, Forsling ML, Poulaint DA (1982) The milk ejection reflex in the pig. J Physiol 333:577–594
Esposito G, Manian N, Truzzi A, Bornstein MH (2017) Response to infant cry in clinically depressed and non-depressed mothers. PLoS One 12:1–15. https://doi.org/10.1371/journal.pone.0169066
Febo M, Numan M, Ferris CF (2005) Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci 25:11637–11644. https://doi.org/10.1523/JNEUROSCI.3604-05.2005
Febo M, Stolberg TL, Numan M, Bridges RS, Kulkarni P, Ferris CF (2008) Nursing stimulation is more than tactile sensation: it is a multisensory experience. Horm Behav 54:330–339. https://doi.org/10.1016/j.yhbeh.2008.02.024
Febo M, Shields J, Ferris CF, King JA (2009) Oxytocin modulates unconditioned fear response in lactating dams: an fMRI study. Brain Res 1302:183–193. https://doi.org/10.1016/j.brainres.2009.09.043
Feldman R (2016) The neurobiology of human attachments. Trends Cogn Sci 21:80–99. https://doi.org/10.1016/j.tics.2016.11.007
Ferris CF, Kulkarni P, Sullivan JM Jr, Harder JA, Messenger TL, Febo M (2005) Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci 25:149–156. https://doi.org/10.1523/JNEUROSCI.3156-04.2005
Fineberg SK, Ross DA (2017) Oxytocin and the social brain. Biol Psychiatry 81:e19–e21. https://doi.org/10.1016/j.biopsych.2016.11.004
Freund-Mercier MJ, Moos F, Poulain DA, Richard P, Rodriguez F, Theodosis DT, Vincent JD (1988) Role of central oxytocin in the control of the milk ejection reflex. Brain Res Bull 20:737–741. https://doi.org/10.1016/0361-9230(88)90085-8
Froemke RC (2015) Plasticity of cortical excitatory-inhibitory balance. Annu Rev Neurosci 38:195–219. https://doi.org/10.1146/annurev-neuro-071714-034002
Fukuoka T, Negoro H, Honda K, Higuchi T, Nishida E (1984) Spinal pathway of the milk-ejection reflex in the rat. Biol Reprod 81:74–81
Galindo-Leon EE, Lin FG, Liu RC (2009) Inhibitory Plasticity in a Lateral Band Improves Cortical Detection of Natural Vocalizations. Neuron 62(5):705–716. https://www.ncbi.nlm.nih.gov/pubmed/19524529. Accessed 11 May 2018
Geerling JC, Loewy AD (2006) Aldosterone-sensitive neurons in the nucleus of the solitary tract: Efferent projections. J Comp Neurol 497(2):223–250. https://www.ncbi.nlm.nih.gov/pubmed/16933386. Accessed 11 May 2018
German RZ, Crompton AW, Hertweck DW, Thexton AJ (1997) Determinants of rhythm and rate in suckling. J Exp Zool 278:1–8. https://doi.org/10.1002/(SICI)1097-010X(19970501)278:1<1::AID-JEZ1>3.0.CO;2-T
Gilbert CD, Li W (2012) Adult visual cortical plasticity. Neuron 75:250–264. https://doi.org/10.1016/j.neuron.2012.06.030
Gilbert CL, Goode JA, McGrath TJ (1994) Pulsatile secretion of oxytocin during parturition in the pig: temporal relationship with fetal expulsion. J Physiol 475:129–137. https://doi.org/10.1113/jphysiol.1994.sp020054
Grinevich FAV (2017) Diversity of oxytocin neurons: beyond magno- and parvocellular cell types? J Neuroendocrinol 0–3. https://doi.org/10.1111/ijlh.12426
Grota LJ, Ader R (1974) Behavior of lactating rats in a dual-chambered maternity cage. Horm Behav 5:275–282. https://doi.org/10.1016/0018-506X(74)90014-2
Hamilton WL, Diamond MC, Johnson RE, Ingham CA (1977) Effects of pregnancy and differential environments on rat cerebral cortical depth. Behav Biol 19:333–340. https://doi.org/10.1016/S0091-6773(77)91674-1
Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K (1991a) The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav 39:71–77. https://doi.org/10.1016/0091-3057(91)90399-M
Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K (1991b) Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci 105:588–598. https://doi.org/10.1037/0735-7044.105.4.588
Hensch TK (2005) Critical period plasticity in local cortical circuits. Nat Rev Neurosci 6:877–888. https://doi.org/10.1038/nrn1787
Hung L, Neuner S, Plepalli J, Beier K, Wright M, Walsh J, Luo L, Deisseroth K, Dölen G, Melenka R (2017) Gating of social reward by oxytocin in the ventral tegmental area. Nature 1411:1406–1411. https://doi.org/10.1126/SCIENCE.AAN4994. https://www.ncbi.nlm.nih.gov/pubmed/28963257. Accessed 11 May 2018
Insel TR, Young LJ (2001) The neurobiology of attachment. Nat Rev Neurosci 2:129–136
Jans JE, Woodside B (1987) Effects of litter age, litter size, and ambient temperature on the milk ejection reflex in lactating rats. Dev Psychobiol 20:333–344. https://doi.org/10.1002/dev.420200310
Johns JM, Joyner PW, McMurray MS, Elliott DL, Hofler VE, Middleton CL, Knupp K, Greenhill KW, Lomas LM, Walker CH (2005) The effects of dopaminergic/serotonergic reuptake inhibition on maternal behavior, maternal aggression, and oxytocin in the rat. Pharmacol Biochem Behav 81:769–785. https://doi.org/10.1016/j.pbb.2005.06.001
Johnson CS, Bains JS, Watts AG (2018) Neurotransmitter diversity in pre-synaptic terminals located in the parvicellular neuroendocrine paraventricular nucleus of the rat and mouse hypothalamus. J Comp Neurol :1287–1306. https://doi.org/10.1002/cne.24407
Jonas K, Johansson LM, Nissen E, Ejdebäck M, Ransjö-Arvidson AB, Uvnäs-Moberg K (2009) Effects of intrapartum oxytocin administration and epidural analgesia on the concentration of plasma oxytocin and prolactin, in response to suckling during the second day postpartum. Breastfeed Med 4(2):71-82. https://doi.org/10.1089/bfm.2008.0002. https://www.ncbi.nlm.nih.gov/pubmed/
Jourdain P, Israel JM, Dupouy B, Oliet SH, Allard M, Vitiello S, Theodosis DT, Poulain DA (1998) Evidence for a hypothalamic oxytocin-sensitive pattern-generating network governing oxytocin neurons in vitro. J Neurosci 18:6641–6649
Keer SE, Stern JM (1999) Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav 67:659–669. https://doi.org/10.1016/S0031-9384(99)00116-X
Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE (2011) Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry Allied Discip 52:907–915. https://doi.org/10.1111/j.1469-7610.2011.02406.x
Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L (2014) Oxytocin and postpartum depression: delivering on what’s known and what’s not. Brain Res 1580:219–232. https://doi.org/10.1016/j.brainres.2013.11.009
Kim P, Strathearn L, Swain JE (2016) The maternal brain and its plasticity in humans. Horm Behav 77:113–123. https://doi.org/10.1016/j.yhbeh.2015.08.001
Knobloch HS, Charlet A, Hoffmann L, Eliava M, Khrulev S, Cetin A, Osten P, Schwarz M, Seeburg P, Stoop R, Grinevich V (2012) Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron 73:553–566. https://doi.org/10.1016/j.neuron.2011.11.030
Kohl J, Dulac C (2018) Neural control of parental behaviors. Curr Opin Neurobiol 49:116–122. https://doi.org/10.1016/j.conb.2018.02.002
Kurth E, Kennedy HP, Zemp Stutz E, Kesselring A, Fornaro I, Spichiger E (2014) Responding to a crying infant—you do not learn it overnight: a phenomenological study. Midwifery 30:742–749. https://doi.org/10.1016/j.midw.2013.06.017
Lebrun CJ, Poulain DA (1982) Electrical activity of septal neurones during suckling and the milk ejection reflex in the lactating rat. Exp Brain Res 47(2):203–208. https://www.ncbi.nlm.nih.gov/pubmed/?term=lebrun+poulain+1982. Accessed 11 May 2018
Lebrun CJ, Poulain DA, Theodosis DT (1983) The role of the septum in the control of the milk ejection reflex in the rat: effects of lesions and electrical stimulation. J Physiol 339:17–31. https://doi.org/10.1113/jphysiol.1983.sp014699
Leng G, Brown CH, Bull PM, Brown D, Scullion S, Currie J, Blackburn-Munro RE, Feng J, Onaka T, Verbalis JG, Russell JA, Ludwig M (2001) Responses of magnocellular neurons to osmotic stimulation involves coactivation of excitatory and inhibitory input: an experimental and theoretical analysis. J Neurosci 21:6967–6977
Lenschow C, Copley S, Gardiner JM, Talbot ZN, Vitenzon A, Brecht M (2016) Sexually monomorphic maps and dimorphic responses in rat genital cortex. Curr Biol 26:106–113. https://doi.org/10.1016/j.cub.2015.11.041
Lenschow C, Sigl-Glöckner J, Brecht M (2017) Development of rat female genital cortex and control of female puberty by sexual touch. PLoS Biol 15:1–22. https://doi.org/10.1371/journal.pbio.2001283
Lin FG, Galindo-Leon EE, Ivanova TN, Mappus RC, Liu RC (2013) A role for maternal physiological state in preserving auditory cortical plasticity for salient infant calls. Neuroscience 247:102–116
Lincoln DW, Wakerley JB (1975) Factors governing the periodic activation of supraoptic and paraventricular neurosecretory cells during suckling in the rat. J Physiol 250:443–461
Lincoln DW, Hentzen K, Hin T, van der Schoot P, Clarke G, Summerlee AJS (1980) Sleep: a prerequisite for reflex milk ejection in the rat. Exp Brain Res 38:151–162. https://doi.org/10.1007/BF00236736
Lingle S, Wyman MT, Kotrba R, Teichroeb LJ, Romanow CA (2012) What makes a cry a cry? A review of infant distress vocalizations. Curr Zool 58:698–726. https://doi.org/10.1093/czoolo/58.5.698
Liu RC, Schreiner CE (2007) Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol 5:e173
Liu RC, Linden JF, Schreiner CE (2006) Improved cortical entrainment to infant communication calls in mothers compared with virgin mice. Eur J Neurosci 23(11):3087–3097
Lonstein JS, Gammie SC (2002) Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev 26:869–888
Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS (2002) A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry 51:431–445. https://doi.org/10.1016/S0006-3223(01)01284-7
Lucas A, Mitchell MD, Drewett RB (1980) Breast-feeding and plasma oxytocin concentrations. Br Med J 281:834–835. https://doi.org/10.1136/bmj.281.6244.834
Ludwig M, Leng G (2006) Dendritic peptide release and peptide-dependent behaviours. Nat Rev Neurosci 7:126–136. https://doi.org/10.1038/nrn1845
Marlin BJ, Mitre M, D’Amour JA, Chao MV, Froemke RC (2015) Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature 520(7548):499–504
Matthiesen AS, Ransjö-Arvidson AB, Nissen E, Uvnäs-Moberg K (2001) Postpartum maternal oxytocin release by newborns: effects of infant hand massage and sucking. Birth 28:13–19. https://doi.org/10.1046/j.1523-536x.2001.00013.x
McNeilly AS, Robinson IC, Houston MJ, Howie PW (1983) Release of oxytocin and prolactin in response to suckling. Br Med J (Clin Res Ed) 286:257–259
Meddle SL, Leng G, Selvarajah JR, Bicknell RJ, Russell JA (2000) Direct pathways to the supraoptic nucleus from the brainstem and the main olfactory bulb are activated at parturition in the rat. Neuroscience 101:1013–1021. https://doi.org/10.1016/S0306-4522(00)00300-6
Michalska KJ, Decety J, Liu C, Chen Q, Martz ME, Jacob S, Hipwell AE, Lee SS, Chronis-Tuscano A, Waldman ID, Lahey BB (2014) Genetic imaging of the association of oxytocin receptor gene (OXTR) polymorphisms with positive maternal parenting. Front Behav Neurosci 8:1–10. https://doi.org/10.3389/fnbeh.2014.00021
Mitre M, Marlin BJ, Schiavo JK, Morina E, Norden SE, Hackett TA, Aoki XCJ, Chao MV, Froemke RC (2016) A distributed network for social cognition enriched for oxytocin receptors. J Neurosci 36:2517–2535
Montoya JL, Landi N, Kober H, Worhunsky PD, Rutherford HJV, Mencl WE, Mayes LC, Potenza MN (2012) Regional brain responses in nulliparous women to emotional infant stimuli. PLoS One 7:1–11. https://doi.org/10.1371/journal.pone.0036270
Moos FC (1995) GABA-induced facilitation of the periodic bursting activity of oxytocin neurones in suckled rats. J Physiol 488:103–114. https://doi.org/10.1113/jphysiol.1995.sp020949
Murray L, Fiori-Cowley A, Hooper R, Cooper P (1996) The impact of postnatal depression and associated adversity on early mother-infant interactions and later infant outcome. Child Dev 67:2512–2526. https://doi.org/10.1111/j.1467-8624.1996.tb01871.x
Nakajima M, Görlich A, Heintz N (2014) Oxytocin modulates female sociosexual behavior through a specific class of prefrontal cortical interneurons. Cell 159:295–305. https://doi.org/10.1016/j.cell.2014.09.020
Newman JD (2007) Neural circuits underlying crying and cry responding in mammals. Behav Brain Res 182:155–165. https://doi.org/10.1016/j.bbr.2007.02.011
Ninan I (2011) Oxytocin suppresses basal glutamatergic transmission but facilitates activity-dependent synaptic potentiation in the medial prefrontal cortex. J Neurochem 119:324–331. https://doi.org/10.1111/j.1471-4159.2011.07430.x
Nishimori K, Young L, Guo Q, Wang Z, Insel T, Matzuk M (1996) Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. PNAS 93:11699–11704
Nissen E, Lilja G, Widstrom AM, Uvnäs-Moberg K (1995) Elevation of oxytocin levels early post partum in women. Acta Obstet Gynecol Scand 74:530–533
Numan M (2007) Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol 49(1):12–21. https://www.ncbi.nlm.nih.gov/pubmed/?term=Numan+M+(2007)+Motivational+systems. Accessed 11 May 2018
Numan M, Young LJ (2016) Neural mechanisms of mother-infant bonding and pair bonding: similarities, differences, and broader implications. Horm Behav 77:98–112. https://doi.org/10.1016/j.yhbeh.2015.05.015
O’Byrne KT, Ring JP, Summerlee AJ (1986) Plasma oxytocin and oxytocin neurone activity during delivery in rabbits. J Physiol 370:501–513. https://www.ncbi.nlm.nih.gov/pubmed/?term=O%E2%80%99Byrne+K%2C+Ring+J%2C+Summerlee+A. Accessed 11 May 2018
Oettl LL, Ravi N, Schneider M, Scheller MF, Schneider P, Mitre M, da Silva Gouveia M, Froemke RC, Chao MV, Young WS, Meyer-Lindenberg A, Grinevich V, Shusterman R, Kelsch W (2016) Oxytocin enhances social recognition by modulating cortical control of early olfactory processing. Neuron 90:609–621. https://doi.org/10.1016/j.neuron.2016.03.033
Olazábal DE, Pereira M, Agrati D, Ferreira A, Fleming AS, González-mariscal G, Lévy F, Lucion AB, Morrell JI, Numan M (2013) Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neurosci Biobehav Rev 37:1875–1892. https://doi.org/10.1016/j.neubiorev.2013.04.004
Onaka T, Luckman SM, Antonijevic I, Palmer JR, Leng G (1995) Involvement of the noradrenergic afferents from the NTS to the SON in OT release after peripheral CCK octapeptide in the rat. Neuroscience 66:403–412
Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW (2013) Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature 500:458–462. https://doi.org/10.1038/nature12330
Paisley AC, Summerlee AJ (1984) Activity of putative oxytocin neurones during reflex milk ejection in conscious rabbits. J Physiol 347:465–478. https://www.ncbi.nlm.nih.gov/pubmed/6707964. Accessed 11 May 2018
Parsons CE, Young KS, Petersen MV, Jegindoe Elmholdt EM, Vuust P, Stein A, Kringelbach ML (2017) Duration of motherhood has incremental effects on mothers’ neural processing of infant vocal cues: a neuroimaging study of women. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-01776-3
Pearson RM, Lightman SL, Evans J (2011) The impact of breastfeeding on mothers’ attentional sensitivity towards infant distress. Infant Behav Dev 34:200–205. https://doi.org/10.1016/j.infbeh.2010.12.009
Poulain DA, Rodriguez F, Ellendorff F (1981) Sleep is not a prerequisite for the milk ejection reflex in the pig. Exp Brain Res 43:107–110. https://doi.org/10.1007/BF00238817
Raby WN, Renaud LP (1989) Dorsomedial medulla stimulation activates rat supraoptic oxytocin and vasopressin neurones through different pathways. J Physiol 417:279–294. https://www.ncbi.nlm.nih.gov/pubmed/?term=raby+renaud+1989. Accessed 11 May 2018
Randle, Bourque R (1986) Alpha1 adrenergic receptor activation depolarizes rat supraoptic neurosecretory neurons in vitro. Am J Phys 251:569–574
Rickenbacher E, Perry RE, Sullivan RM, Moita MA (2017). Freezing suppression by oxytocin in central amygdala allows alternate defensive behaviours and mother-pup interactions. Elife (accepted, 1–17) https://doi.org/10.7554/eLife.24080
Riem MME, Pieper S, Out D, Bakermans-Kranenburg MJ, van Ijzendoorn MH (2011) Oxytocin receptor gene and depressive symptoms associated with physiological reactivity to infant crying. Soc Cogn Affect Neurosci 6:294–300. https://doi.org/10.1093/scan/nsq035
Rilling JK, Young LJ (2014) The biology of mammalian parenting and its effect on offspring social development. Science 345(80):771–776. https://doi.org/10.1126/science.1252723
Rogers-Carter MM, Varela JA, Gribbons KB, Pierce AF, McGoey MT, Ritchey M, Christianson JP (2018) Insular cortex mediates approach and avoidance responses to social affective stimuli. Nat Neurosci 21:404–414. https://doi.org/10.1038/s41593-018-0071-y
Rosselet C, Zennou-Azogui Y, Xerri C (2006) Nursing-induced somatosensory cortex plasticity: temporally decoupled changes in neuronal receptive field properties are accompanied by modifications in activity-dependent protein expression. J Neurosci 26:10667–10676. https://doi.org/10.1523/JNEUROSCI.3253-06.2006
Rothschild G, Cohen L, Mizrahi A, Nelken I (2013) Elevated correlations in neuronal ensembles of mouse auditory cortex following parturition. J Neurosci 33:12851–12861. https://doi.org/10.1523/JNEUROSCI.4656-12.2013
Sabihi S, Dong SM, Durosko NE, Leuner B (2014) Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Front Behav Neurosci 8:1–11. https://doi.org/10.3389/fnbeh.2014.00258
Sander K, Scheich H (2005) Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci 17:1519–1531. https://doi.org/10.1162/089892905774597227
Shepard KN, Lin FG, Zhao CL, Chong KK, Liu RC (2015) Behavioral relevance helps untangle natural vocal categories in a specific subset of Core auditory cortical pyramidal neurons. J Neurosci 35:2636–2645. https://doi.org/10.1523/JNEUROSCI.3803-14.2015
Shepard KN, Chong KK, Liu RC (2016) Contrast enhancement without transient map expansion for species-specific vocalizations in core auditory cortex during learning. eNeuro 3. https://doi.org/10.1523/ENEURO.0318-16.2016
Silva MR, Bernardi MM, Felicio LF (2001) Effects of dopamine receptor antagonists on ongoing maternal behavior in rats. Pharmacol Biochem Behav 68:461–468. https://doi.org/10.1016/S0091-3057(01)00471-3
Stern JM, Taylor LA (1991) Haloperidol inhibits maternal retrieval and licking, but enhances nursing behavior and litter weight gains in lactating rats. J Neuroendocrinol 3:591–596. https://doi.org/10.1111/j.1365-2826.1991.tb00323.x
Stoop R (2012) Neuromodulation by oxytocin and vasopressin. Neuron 76:142–159. https://doi.org/10.1016/j.neuron.2012.09.025
Summerlee AJ (1981) Extracellular recordings from oxytocin neurones during the expulsive phase of birth in unanaesthetized rats. J Physiol 321:1–9. https://doi.org/10.1113/jphysiol.1981.sp013967
Summerlee AJS, Paisley AC (1982) The effect of behavioural arousal on the activity of hypothalamic neurons in unanaesthetized, freely moving rats and rabbits. Proc R Soc London Ser B, Biol Sci 214:263–272
Swain JE, Tasgin E, Mayes LC, Feldman R, Todd Constable R, Leckman JF (2008) Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry Allied Discip 49:1042–1052. https://doi.org/10.1111/j.1469-7610.2008.01963.x
Swain JE, Kim P, Ho SS (2011) Neuroendocrinology of parental response to baby-cry. J Neuroendocrinol 23:1036–1041. https://doi.org/10.1111/j.1365-2826.2011.02212.x
Tang Y, Chen Z, Tao H, Li C, Zhang X, Tang A, Liu Y (2014) Oxytocin activation of neurons in ventral tegmental area and interfascicular nucleus of mouse midbrain. Neuropharmacology 77:277–284. https://doi.org/10.1016/j.neuropharm.2013.10.004
Tasaka GI, Guenthner CJ, Shalev A, Gilday O, Luo L, Mizrahi A (2018) Genetic tagging of active neurons in auditory cortex reveals maternal plasticity of coding ultrasonic vocalizations. Nat Commun 9. https://doi.org/10.1038/s41467-018-03183-2
Tay CCK, Glasier AF, Mcneilly AS (1993) Effect of antagonists of dopamine and opiates on the basal and GnRH-induced secretion of luteinizing hormone, follicle stimulating hormone and prolactin during lactational amenorrhoea in breastfeeding women. Hum Reprod 8:532–539. https://doi.org/10.1093/oxfordjournals.humrep.a138090
Thornton S, Davison JM, Baylis PH (1988) Plasma oxytocin during third stage of labour: comparison of natural and active management. BMJ 297:167–169. https://doi.org/10.1136/bmj.297.6642.167
Weitzman RE, Leake RD, Rubin RT, Fisher DA (1980) The effect of nursing on neurohypophyseal hormone and prolactin secretion in human subjects. J Clin Endocrinol Metab 51:836–839
Westneat MW, Hall WG (1992) Ontogeny of feeding motor patterns in infant rats: an electromyographic analysis of suckling and chewing. Behav Neurosci 106:539–554. https://doi.org/10.1037/0735-7044.106.3.539
Xerri C, Stern JM, Merzenich MM (1994) Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci 14:1710–1721. https://doi.org/10.1038/nn1800
Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y, Xiao L, Priest MF, Nasenbeny J, Lu T, Kozorovitskiy Y (2017) Biased oxytocinergic modulation of midbrain dopamine systems article biased oxytocinergic modulation of midbrain dopamine systems. Neuron 95:368–384, e5. https://doi.org/10.1016/j.neuron.2017.06.003. https://www.ncbi.nlm.nih.gov/pubmed/?term=xiao+oxytocin+elife+2018
Xiao L, Priest MF, Kozorovitskiy Y (2018) Oxytocin functions as a spatiotemporal filter for excitatory synaptic inputs to VTA dopamine neurons. Elife 7. https://doi.org/10.7554/eLife.33892
Yamashita H, Inenaga K, Kannan H (1987) Depolarizing effect of noradrenaline on neurons of the rat supraoptic nucleus in vitro. Brain Res 4:348–352
Zeifman D (2011) An ethological analysis of human infant crying: answering Tinbergen’ s four questions. Dev Psychobiol 39:265–285
Zheng JJ, Li SJ, Di Zhang X, Miao WY, Zhang D, Yao H, Yu X (2014) Oxytocin mediates early experience-dependent cross-modal plasticity in the sensory cortices. Nat Neurosci 17:391–399. https://doi.org/10.1038/nn.3634
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Valtcheva, S., Froemke, R.C. Neuromodulation of maternal circuits by oxytocin. Cell Tissue Res 375, 57–68 (2019). https://doi.org/10.1007/s00441-018-2883-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-018-2883-1