Abstract
Preferential degeneration of dopamine neurons (DAn) in the midbrain represents the principal hallmark of Parkinson’s disease (PD). It has been hypothesized that major contributors to DAn vulnerability lie in their unique cellular physiology and architecture, which make them particularly susceptible to stress factors. Here, we report a concise overview of some of the cell mechanisms that may exacerbate DAn sensitivity and loss in PD. In particular, we highlight how defective protein sorting and clearance, endoplasmic reticulum stress, calcium dyshomeostasis and intracellular trafficking converge to contribute synergistically to neuronal dysfunction in PD pathogenesis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
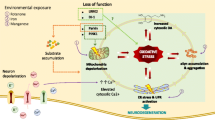
Parkinson’s disease (PD) is one of the most common age-related neurodegenerative disorders, characterized clinically by a progressive appearance of motor deficits that include resting tremor, muscular rigidity, bradykinesia, postural abnormalities and instability (Jankovic 2008). The underlying cause of PD is often attributed to an interplay between environmental and genetic factors (Horowitz and Greenamyre 2010); however, the majority of the cases are idiopathic and the underlying etiology remains largely elusive. Significant advances in understanding the mechanisms of disease pathogenesis have been made in the past two decades with the identification of pathogenic mutations associated with parkinsonism, although these genetic anomalies only account for 5–10% of PD patients (Corti et al. 2011). Whether it is the sporadic or hereditary form, the common feature is the preferential loss of dopamine neurons (DAn) of the substantia nigra (SN) projecting to the striatum (Damier et al. 1999). These neurons show unique cellular features that make them more susceptible than other neuronal populations in the brain. Indeed, their extensive and unmyelinated axonal innervation conveys an exceptionally high energy cost that makes them more vulnerable to any perturbation of energy supply (Bolam and Pissadaki 2012). Moreover, alteration of different cellular mechanisms such as protein degradation pathways, endoplasmic reticulum (ER) function, calcium signaling and intracellular trafficking enhance the vulnerability of DAn converging towards their progressive degeneration (Fig.1).
Convergent pathways towards dopaminergic neurodegeneration in PD. Combination of genetics and environmental factors exacerbate selective vulnerability of DAn by dysregulation of related cellular pathways including proteostatic dysfunction, ER stress, rupture of intracellular trafficking and alteration of calcium homeostasis
In this review, we discuss recent insights into the study of these aberrant mechanisms and we highlight how some of them share common elements finally contributing to the pathogenesis of PD.
Dysfunctional autophagy/lysosome pathways in PD
Autophagy is a dynamic cellular homeostatic process essential for bulk degradation of cytoplasmic contents and constitutes the main proteolytic system in neurons (Nikoletopoulou et al. 2015). According to the pathway by which the autophagic cargo is delivered within the lysosomes, three types of autophagy can be distinguished: microautophagy, macroautophagy and chaperone-mediated autophagy (CMA) (Cuervo 2010). Microautophagy takes place when cytosolic components are directly internalized through invaginations at the lysosome membrane and, once in the lysosomal lumen, the whole internalized vesicle is degraded (Kunz et al. 2004). In contrast, during macroautophagy, the sequestration of cytosolic cargo is triggered by a double-membrane phagophore that expands into a vesicle called autophagosome. Subsequently, the autophagosome fuses with lysosomes to allow the cargo to be degraded and recycled (Klionsky 2005). CMA differs from the other two autophagic pathways since it does not involve vesicle formation and the degradation is based on the recognition of a specific amino acid sequence (KFERQ). In this case, the cargo, which is recognized by the cytosolic heat shock cognate protein 70 (Hsc70), directly translocates into the lysosome across the lysosomal membrane with the help of a set of lysosomal proteins such as the lysosome-associated membrane protein type 2A (LAMP2A) (Kon and Cuervo 2010).
Autophagy is generally considered to exert a neuroprotective role (Dohi et al. 2012; Rodriguez-Muela and Boya 2012; Jeong et al. 2013) and growing evidence indicates that its down-regulation leads to the accumulation of aberrant proteins as inclusion bodies contributing to the pathogenesis of neurodegenerative disorders (Hara et al. 2006; Komatsu et al. 2006). Indeed, as neuronal cells are more sensitive to accumulation of toxic components than dividing ones, high activity of the intracellular protein degradation systems appears to be crucial in order to maintain neuronal function and prevent cell death. The presence of intraneuronal protein inclusions within the brain stem (Spillantini et al. 1997) supports a role for autophagic failure in PD. These insoluble protein aggregates, known as Lewy bodies (LBs), are principally composed of α-synuclein (αSyn), a normally presynaptic protein with a physiological function related to neurotransmitter release at the nerve terminal (Bendor et al. 2013). In its native state, αSyn is typically unfolded (Weinreb et al. 1996); however, the protein is extremely sensitive to its environment and can change its conformation to monomeric and oligomeric states, leading to misfolding and aggregation during the process (Uversky 2007). CMA and macroautophagy are responsible for αSyn degradation and impairment of either of these two pathways has been related to its pathogenic accumulation in PD (Vogiatzi et al. 2008) (Fig. 2). CMA-related proteins, Hsc70 and LAMP2A, were found reduced in the SN and amygdala of post-mortem brain from PD patients (Alvarez-Erviti et al. 2010), providing additional evidence for altered CMA activity in PD. Moreover, LAMP2A reduction is correlated with increased αSyn and decreased levels of Hsc70 in the early stages of PD (Murphy et al. 2015), suggesting that CMA dysregulation occurs before substantial αSyn aggregation in PD. The involvement of dysfunctional macroautophagy in αSyn accumulation was demonstrated by the finding that specific deletion of the autophagy-related gene 7 (Atg7) results in accumulation of αSyn aggregates together with loss of dopaminergic neurons and reduced striatal dopamine content (Ahmed et al. 2012; Friedman et al. 2012). Accumulating evidence also suggests that αSyn and its pathogenic forms can exert inhibitory effects on the degradation pathways. For instance, αSyn overexpression can inhibit autophagosome biogenesis through interaction with the autophagy regulator Rab1a (Winslow et al. 2010). Association of αSyn with the transcription factor EB (TFEB), a critical regulator of lysosomal biogenesis, autophagy and lysosomal degradation, has been recently suggested as a mechanism underlying autophagy/lysosomal pathway dysfunction. Indeed, high cellular levels of αSyn in nigral DAn block the translocation of TFEB to the nucleus, leading to a depletion of markers of lysosome function (Decressac et al. 2013). In addition, a recent study reported that αSyn aggregates are able to resist macroautophagy clearance, leading to a failure of the pathway and accumulation of autophagosomes (Tanik et al. 2013). Furthermore, two αSyn familial mutations, SNCA-A30P and SNCA-A53T, have been involved in the impairment of the CMA pathway (Cuervo et al. 2004). Both mutant forms of the protein exhibited higher affinity to the LAMP2A receptor than the wild-type form but failed to be degraded, favoring toxic gains-of-functions by contributing to its aggregation or additional modifications, such as dopamine-adduct formation (Martinez-Vicente et al. 2008), which further underlie PD DAn vulnerability.
Dysfunctional cellular mechanisms in PD. Convergent molecular mechanisms in protein homeostasis, intracellular trafficking, the autophagic/lysosomal pathway and calcium signaling in Parkinson’s. Aggregation of misfolded αSyn is responsible for ER stress and failure of autophagic pathways and sequestration of Hsc70 into the lysosome. Impairment of macroautophagy machinery is attributable to mutations in SNCA, Atg7, ATP13A2 and LRRK2. Association of αSyn transcription factor TFEB blocks TFEB translocation into the nucleus, hence impairing the expression of autophagy/lysosome-related genes. αSyn aggregates inhibit ER-Golgi vesicular transport by interaction with Rab1a and impair endosomal transport and fusion with lysosome by disrupting Rab7 function. PD-related mutations in DNAJC13, DNAJC6 and GAK induce perturbation in endosomal trafficking and disrupt clathrin mediated endocytocis. Parkinson’s-associated mutations lead to Ca2+ mishandling and Ca2+ dyshomeostasis through alteration of ER-mitochondrial tethering, reducing Ca2+ transport between organelles and a failure of intracellular buffering mechanisms. Sustained Cav channel opening underlies low intrinsic buffering capacity dopaminergic neurons leading to elevated Ca2+ influx
Besides αSyn, other PD-related genes have been shown to contribute to the autophagy failure. One of them is the leucine-rich repeat kinase 2 (LRRK2) in which autosomal-dominant mutations represent the major common monogenic forms of familial PD (Zimprich et al. 2004; Lubbe and Morris 2014). LRRK2 protein localizes in the cytosol as well as to specific membrane domains, including mitochondria and autophagosomes (Biskup et al. 2006; Alegre-Abarrategui et al. 2009). Given its widespread localization, LRRK2 has been associated with several cellular functions and signaling pathways, including mitochondrial function and vesicle trafficking together with endocytosis and autophagy (Alegre-Abarrategui and Wade-Martins 2009; Wallings et al. 2015). Recent findings have shown that two of the most frequent mutations of LRRK2, G2019S and R1441C, result in progressive degeneration of dopaminergic neurons due to the accumulation of autophagic vacuoles and increased mitochondrial autophagy (Ramonet et al. 2011). In addition, primary fibroblasts carrying these PD-related pathogenic mutations exhibit alterations in markers for autophagy/lysosomal function (Manzoni et al. 2013), highlighting a role for LRRK2 in the dysfunction of the autophagy/lysosomal pathway in PD. In accordance with these findings, it has recently been demonstrated that LRRK2 regulates lysosome size, number and function and that expression of PD-associated LRRK2 variants results in enlarged lysosomes and reduces the lysosomal capacity of the cell (Henry et al. 2015). Moreover, DAn differentiated from induced pluripotent stem cells (iPSCs) generated from PD patients carrying familial LRRK2 mutations showed accumulation of autophagic vacuoles that occurs after defective autophagosome clearance (Sanchez-Danes et al. 2012). Interestingly, this impairment in autophagy is associated with abnormal accumulation of αSyn, suggesting that the two proteins may act synergistically to induce neurodegeneration and that LRRK2 can accelerate mutant αSyn-induced neuropathology as previously reported in mice in a dose-dependent manner (Lin et al. 2009). Further evidence supporting this hypothesis comes from another study that reported that pathogenic LRRK2 may promote oligomerization of αSyn on the lysosomal surface, inhibiting its uptake into the lysosome and CMA-mediated degradation (Orenstein et al. 2013) (Fig. 2).
Autophagic perturbations with subsequent accumulation of αSyn have also been observed when glucocerebrosidase (GCase) activity is compromised. GCase is a lysosomal enzyme encoded by the GBA gene and mutations are recognized as an important genetic risk factor for the development of PD (Sidransky et al. 2009). In studies conducted in neurons derived from iPSC lines carrying either partial depletion of GCase or common mutated forms (GBA-N370S and GBA-L444P) demonstrate impairment of lysosomal protein degradation and a substantial increase of αSyn levels (Mazzulli et al. 2011; Schondorf et al. 2014; Fernandes et al. 2016). Importantly, experiments conducted in brain tissue from patients with sporadic PD confirm that GCase activity is also reduced without GBA mutations and is associated with lysosomal dysfunction and accumulation of αSyn (Murphy et al. 2014). Recently, it has been proposed that these detrimental events following GCase deficiency are due to the impairment of lysosomal recycling and endosome maturation processes (Magalhaes et al. 2016) (Fig. 2). Accordingly, mutations in other genes encoding proteins of endosomal/lysosomal processes such as the cation-transporting ATPase (ATP13A2) result in PD-like neuropathology through defective autophagy. The ATP13A2 gene (also known as PARK9) is mutated in autosomal recessive forms of early-onset Parkinsonism with pyramidal degeneration and dementia (Ramirez et al. 2006). Experiments conducted in ATP13A2 PD patient-derived fibroblasts showed that ATP13A2 loss of function leads to several lysosomal alterations including impaired lysosomal acidification, decreased proteolytic processing of lysosomal enzymes, reduced degradation of lysosomal substrates and diminished lysosomal-mediated clearance of autophagosomes (Dehay et al. 2012). Moreover, ATP13A2 deficiency drives neurotoxicity through the accumulation of αSyn (Usenovic et al. 2012). Importantly, recent findings highlight that ATP13A2 may be capable of regulating synaptotagmin 11 (SYT11), another protein emerging with a function in PD (Usenovic et al. 2012). Knockdown of ATP13A2 decreases SYT11 transcription, which in turn blocks autophagosome clearance and compromises lysosomal function (Bento et al. 2016). Thus, defects in the ATP13A2/SYT11 network are likely to contribute to lysosomal dysfunction, autophagy blockage and impairment of αSyn clearance associated with PD. Finally, Lmx1b has been recently identified as a crucial transcription factor involved in the maintenance of normal autophagic/lysosomal and intracellular transport functions and its dysfunction has been tightly linked to the onset of PD pathology (Laguna et al. 2015). Expression of the transcription factors, Lmx1a and Lmx1b, is known to be necessary for the development of mid-brain DA neurons (Yan et al. 2011). Laguna and collaborators found that their activity persists even after the neurons have matured. Moreover, they observed reduced levels of Lmx1b in the SN DA neurons of PD brain tissue (Fig. 2), together with an alteration in the autophagic/lysosomal pathway clearance systems followed by degenerative loss of SN DA neurons in Lmx1b-ablated animal models.
Defective intracellular trafficking in PD
Intracellular protein trafficking has an important role in the biology of neuronal function and survival. Two major cellular pathways are responsible for shuttling vesicles transport proteins and lipids between the membrane-bounded compartments of the cell and its environment (Tokarev et al. 2009). First, in the exocytic pathway, proteins synthesized in the cytoplasm are translocated into the ER and, from here, membranous vesicles shuttle the cargo to the Golgi apparatus. In the last step, the Golgi apparatus sorts and ships the proteins to their final cellular destinations, such as the plasma membrane for constitutive and regulated secretion but also to endosomes and lysosomes, or back to the ER. Second, in the endocytic pathway, proteins and membrane are internalized at the plasma membrane by either clathrin-dependent or clathrin-independent endocytosis and delivered to the early endosome where sorting occurs. When the endosome has matured into a late endosome, it finally fuses with a lysosome that represents the degradative end-point at which the endosomal and autophagic pathways converge.
Recent findings point to faulty trafficking as contributing to the pathological accumulation and clearance of misfolded proteins, finally leading to dysfunction and degeneration of neurons and neural circuits (Wang et al. 2014). Importantly, advances in genetics and experimental discoveries have highlighted that defects in the intracellular trafficking machinery are involved in the development of PD (Hunn et al. 2015; Abeliovich and Gitler 2016), which may explain the association of the microtubule-associated membrane tau with PD. The high capacity of αSyn to bind to acidic phospholipid vesicles (Jo et al. 2000) suggests a role for αSyn in the regulation of vesicle trafficking and prompts the hypothesis that its pathogenic form may cause dysfunctional intracellular trafficking in PD. For instance, recent studies conducted in PD patient-derived iPSC neurons have shown that the accumulation of αSyn inhibits trafficking of important enzymes to the lysosome by altering the ER-Golgi localization of Rab1a, a key mediator of vesicular transport (Mazzulli et al. 2016) (Fig. 2). Interestingly, upregulation of Rab1 is sufficient to protect against αSyn-induced neuron loss, suggesting that enhancing protein trafficking can reverse the pathogenic link between αSyn and lysosomal dysfunction (Cooper et al. 2006; Mazzulli et al. 2016). Other evidence comes from studies conducted in primary neurons that demonstrated that αSyn aggregates impair Rab7-positive endosomal transport and fusion with lysosomes (Volpicelli-Daley et al. 2014). The familial PD-associated SNCA-A53T mutation in the gene encoding αSyn has also been associated with defects in the ER–Golgi transport, through the inhibition of the fusion of ER vesicles to the Golgi membrane (Gitler et al. 2008; Thayanidhi et al. 2010). The proposed mechanism reveals that A53T αSyn directly binds to ER–Golgi SNAREs, a class of proteins essential for the fusion of vesicles with membranes. This interaction is sufficient to inhibit SNARE complex assembly, reducing the events that lead to membrane fusion and suggesting a potential αSyn-dependent toxic effect on synaptic vesicle exocytic machinery. Importantly, a study conducted in A53T-αSyn transgenic mice showed that A53T αSyn-induced ER–Golgi trafficking defects can be exacerbated by the overexpression of LRRK2, suggesting a synergistic cytotoxic effect that finally leads to the fragmentation of the Golgi apparatus and increased αSyn accumulation in the soma (Lin et al. 2009).
Co-localization of LRRK2 with the late endosomal marker Rab7 in αSyn-positive brainstem LBs implicates LRRK2 in the function of the endo-lysosomal pathway (Higashi et al. 2009). Notably, the expression of mutant LRRK2 in both cell and animal models leads to defective late endosome maturation and fusion with lysosomes by impairing the interaction with Rab7 and its function (Dodson et al. 2012; Gomez-Suaga et al. 2014). Recent phosphoproteomic screens have revealed that one of the key functions of LRRK2 kinase activity is to regulate the activity of proteins from the Rab family and, consequently, vesicular trafficking events (Steger et al. 2016). Indeed, apart from Rab7, LRRK2 has been shown to interact with other Rab proteins including Rab29/Rab7L1, a Golgi-resident Rab encoded by the PARK16 non-familial PD risk-associated locus (MacLeod et al. 2013). In this study, it was reported that knockdown of Rab7L1 recapitulated degeneration observed with the expression of a familial PD mutant form of LRRK2 in rodent or Drosophila dopamine neurons, whereas Rab7L1 overexpression rescued the LRRK2 mutant phenotypes. This neuronal loss is attributable to defective endo-lysosomal and Golgi apparatus sorting defects (Fig. 2). Interestingly, these defects can be rescued by the expression of wild-type VPS35, a component of the retromer complex, which mediates endosome–Golgi retrieval of membrane proteins (Bonifacino and Hurley 2008). Recently, it has been shown that a PD-causing mutation of VPS35 protein induces marked degeneration of dopaminergic neurons (Tsika et al. 2014; Tang et al. 2015) and that defects in autophagy, as well as in the trafficking of lysosomal protein cathepsin D and the transmembrane autophagy protein ATG9A, have also been proposed as putative mechanisms (Follett et al. 2014; Zavodszky et al. 2014). Indeed, mutant VPS35 exhibits reduced association with the WASH complex (McGough et al. 2014), impairing its endosomal recruitment and thus perturbing endosomal/lysosomal trafficking.
Recent investigations on further genes linked to PD have underscored the importance of the endocytic pathway in disease pathogenesis. Among them, mutations in DNAJC13, DNAJC6 and GAK, which encode for functionally related proteins that control clathrin-dependent endocytosis, have been associated with familial and sporadic PD (Fig. 2). In particular, the receptor-mediated endocytosis 8/RME-8 (DNAJC13) regulates the dynamics of clathrin coating on early endosomes and recent studies have reported that the p.Asn855Ser mutation confers a toxic gain-of-function and impairs endosomal transport (Vilarino-Guell et al. 2014). Similarly, mutation in auxilin 1 encoded by DNAJC6 leads to impaired synaptic vesicle recycling and perturbed clathrin-mediated endocytosis by increased retention of assembled clathrin on vesicles and in empty cages (Edvardson et al. 2012). Cyclin G-associated kinase (GAK) plays key roles for clathrin exchange as well as for clathrin uncoating (Lee et al. 2006) and single nucleotide polymorphisms (SNPs) in the GAK locus have been identified as risk factors for sporadic PD by genome-wide association studies (Nalls et al. 2014). Especially, microarray analysis of post-mortem PD and control brains has demonstrated a significant correlation between one of the identified GAK SNPs and increased αSyn expression (Dumitriu et al. 2011). This event is recapitulated when GAK expression is downregulated in cell culture, causing a significant increase in toxicity. Intriguingly, GAK has also been proposed to bind and form a complex with LRRK2 with the function of promoting the clearance of Golgi-derived vesicles (Beilina et al. 2014). Altogether, these genetics findings suggest that variants in different loci converge towards defective trafficking and sorting, culminating in lysosomal dysfunction and aberrant protein degradation.
ER stress in PD
It is well established that ER stress is also a potent trigger of autophagy and the occurrence of chronic ER stress has been extensively described in neurodegenerative conditions, including PD (Ogata et al. 2006; Matus et al. 2008). Importantly, ER stress and autophagy cooperate to protect cells by relieving stress and inducing cell death under extreme conditions, thus alteration of the function of one of these systems can influence the other (Rashid et al. 2015).
The ER is a sensitive sensor of cellular homeostasis and maintains proper protein folding and quality control (McCaffrey and Braakman 2016). Disruption of the folding process and accumulation of misfolded or unfolded proteins in the ER trigger the activation of the unfolded protein response (UPR) to counteract the situation (Hetz 2012). Three main stress sensors recognize unfolded proteins in the ER, including inositol-requiring protein-1 (IRE1), protein kinase RNA-like ER kinase (PERK) and the activating transcription factor-6 (ATF6). The activation mechanism of these proteins has not been completely defined but it is known that molecular chaperones of ER lumen, such as BiP (Grp78), are involved in the activation of these transmembrane transducers (Lee 2005). Many reports suggest the involvement of ER stress in the pathology of PD and accumulation of misfolded αSyn has been proposed as a major cause of this deleterious process. For instance, expression of A53T-αSyn in differentiated PC12 cells showed decreased proteasome activity and increased ER stress (Smith et al. 2005). In another recent study, αSyn overexpression and accumulation inhibited the neuroprotective activity of ATF6, which is normally processed via COPII-mediated ER–Golgi transit following its activation via ER stress (Credle et al. 2015). Lower levels of ATF6 and its reduced incorporation in COPII vesicles were also observed in presence of mutated A53T-αSyn. Impaired ATF6 signaling is accompanied by decreased ER-associated degradation (ERAD) function, which sensitizes cells to apoptosis, thereby disrupting UPR signaling. However, how αSyn could initiate ER stress is still a matter of debate. It has been suggested that αSyn may induce ER stress by disrupting ER–Golgi vesicular trafficking leading to ER overload (Cooper et al. 2006). Moreover, αSyn toxic oligomers may accumulate in the lumen of the ER early during the disease process, with subsequent upregulation of ER chaperones (Colla et al. 2012). Furthermore, activation of the PERK–eIF2α pathway of the UPR occurs concomitantly with αSyn cytoplasmic accumulations in nigral dopaminergic neurons of PD cases (Hoozemans et al. 2007). Increase of the UPR mediators and ER stress also occurs when misfolded GCase is trapped in the ER (Ron and Horowitz 2005). In line with this, iPSC-derived DA neurons carrying the PD-associated GBA-N370S mutation showed activation of UPR with significant upregulation of ER-resident chaperones, such as BiP, IRE1α and PDI (Fernandes et al. 2016). Furthermore, evidence has highlighted the pathogenic role of parkin in regulating the ERAD of misfolded ER proteins. Mutations of the parkin gene, which compromise the ubiquitin ligase function of the protein (Dawson and Dawson 2010), result in the accumulation of its substrates in the ER of SN dopaminergic neurons, leading to ER stress and cell death (Imai et al. 2001).
Apart from the ER stress response associated with aggregation of misfolded proteins, it has been recently shown that downregulation of parkin may increase the vulnerability of cells to ER stress-induced mitochondrial dysfunctions, suggesting an interconnection between mitochondrial and ER stress, with parkin playing a central role in this connection (Bouman et al. 2011) (Fig. 2). In line with this, mutations in parkin and PTEN-induced putative kinase 1 (PINK1) may enhance ER stress signaling through defective ER-mitochondria tethering (Celardo et al. 2016). This deleterious event is due to an increase in contacts between mitochondria and the ER, which is promoted by increased levels of the profusion factor mitofusin. Thus, activation of ER stress seems to be linked to the functional status of mitochondria at ER contacts and disruption of these connections may enhance the vulnerability of DAn in PD. Interestingly, point mutations in αSyn can reduce the number of appositions between ER and mitochondria, thereby altering their function (Guardia-Laguarta et al. 2014). Moreover, downregulation of DJ-1, a protein associated with rare forms of inherited early-onset PD, promotes morphological changes in mitochondria, leading to alteration of the contacts between the two organelles (Ottolini et al. 2013). Impairment of ER–mitochondria tethering finally reduces calcium transfer between the two compartments, indicating the importance of these interactions in order to preserve normal physiology and prevent neurodegeneration.
Calcium signaling in PD
Calcium (Ca2+) serves multiple complex and integrated functions in neurons, including the control of dendritic responses to neurotransmitters, signaling to the nucleus to regulate gene expression and initiation of neurotransmitter release from presynaptic axon terminals (Brini et al. 2014). Ca2+ is a messenger that transfers signals within the cell in response to membrane depolarization, thereby relaying information on neuronal activity status within the neuron. Ca2+ signals are decoded based on the characteristics of the intracellular changes in Ca2+ concentration and generate outputs as different as proliferation or death. Owing to its vital importance, a coordinated system to control Ca2+ concentration is required to guarantee proper neuronal function. This system includes Ca2+-buffering proteins, exchangers, pumps and voltage- and ligand-gated channels in the plasma membrane that regulate active extrusion of the ion to the extracellular space or sequestration into intracellular organelle stores. Neurons express various types of voltage-gated Ca2+ (Cav) channels including the Cav1 or L-type, Cav2 and Cav3 isoforms (Hurley and Dexter 2012). The Cav1 and Cav2 channels activate at high depolarization voltage and produce sustained large Ca2+ currents, whereas the Cav3 channels activate at low voltage, generating transient Ca2+ currents. Importantly, SN dopaminergic neurons differ from many other neuronal populations by having autonomous activity and generating continuous low frequency (2–10 Hz) activity in the absence of conventional synaptic input (Grace and Bunney 1983). This phenomenon is dependent on Cav1-type channels, especially on the pore-forming Cav1.3 subunit, which opens at more hyperpolarized potentials than Cav1.2 channels, thus exposing SN dopaminergic neurons to elevated Ca2+ influx (Xu and Lipscombe 2001; Chan et al. 2007) (Fig. 2). Although this autonomous pacemaking activity serves to maintain a constant dopamine supply in the striatum (Surmeier 2007), it may underlie the preferential vulnerability of SN DA neurons that exhibit low intrinsic Ca2+-buffering capacity (Foehring et al. 2009), in part due to low levels of the protein calbindin. Indeed, recent work has shown that continuous rises in cytosolic Ca2+ occurring in SN DA neurons are able to induce mitochondrial oxidative stress (Guzman et al. 2010). Oxidant stress generates mild mitochondrial depolarization or uncoupling, which leads to bioenergetic deficiency, exacerbating the vulnerability of DAn towards a condition of increased metabolic demand. Increased vulnerability to stress responses involving elevation of cytosolic Ca2+ overload was also observed in iPSC-derived neurons harboring GBA mutations (Schondorf et al. 2014). Dysregulation of calcium homeostasis in GBA mutant neurons was accompanied by an increased expression of the neuronal calcium-binding protein NECAB2, suggesting a compensatory mechanism in such vulnerable neurons. Defects in Ca2+-buffering capacity are also evident in neurons expressing mutant LRRK2. Several studies support a role for LRRK2 in Ca2+ signaling and homeostasis, reporting that mutated forms of the kinase protein generate altered calcium levels along with aberrations in mitochondrial degradation, dendrite shortening and neurite aggregation (Cherra et al. 2013; Schwab and Ebert 2015). In line with this, it has been recently demonstrated that LRRK2 can impact Cav channel function, especially Cav of type 2 (Bedford et al. 2016). Interestingly, the authors found that CaV2.1 activation is dependent on the kinase activity of LRRK2 causing a large increase in Ca2+ current. Moreover, the PD-related G2019S LRRK2 mutation stimulated CaV2.1 channels to a greater degree than the wild-type, supporting the hypothesis that LRRK2 mutations disrupt normal Ca2+ signaling in PD. In addition to LRRK2, other studies have pointed out that αSyn can alter calcium homeostasis, enhancing the voltage-operated Ca2+ channel activity. Indeed, the incubation of differentiated neuroblastoma cell lines and primary rat cortical neurons with a medium containing secreted αSyn induces an increase in capacitive Ca2+ entry and calpain-mediated toxicity (Melachroinou et al. 2013). Accordingly, dysregulation of spontaneous and stimulus-evoked neuronal calcium activity was observed in transgenic mice overexpressing human αSyn (Reznichenko et al. 2012). The augmented Ca2+ transients and defective decay of the Ca2+ peak without any change in the neuronal spiking response suggest that αSyn promoted alterations in Ca2+ dynamics via interference with intracellular buffering mechanisms.
Due to their capacity to buffer high cytosolic calcium levels, functional mitochondria are crucial in order to prevent Ca2+ dyshomeostasis. Evidence for a possible role of mitochondrial Ca2+ mishandling in the pathogenesis of PD comes from studies on the mitochondrial kinase, PINK1. The first suggestion arose from the finding that the expression of mutant PINK1 exacerbates mitochondrial defects in a cellular model of PD. These defects, such as loss of mitochondrial membrane potential, increased mitochondrial size with loss of cristae and reduced ATP levels, are fully rescued by the inhibition of the mitochondria calcium uniporter, suggesting that mitochondrial Ca2+ uptake is involved (Marongiu et al. 2009). Other studies have proposed that the absence of PINK1 induces mitochondrial Ca2+ accumulation, possibly as a consequence of the impairment of mitochondrial Ca2+ efflux through the mitochondrial Na+/Ca2+ exchanger (Gandhi et al. 2009). Depletion of PINK1 could also impair mitochondrial Ca2+ uptake and, consequently, ATP production (Heeman et al. 2011) (Fig. 2). Moreover, increased sensitivity of mitochondria to Ca2+-induced permeability has been shown to precede dopaminergic defects in PINK1-deficient mice, suggesting that mitochondrial Ca2+ alteration could be an early event in the pathogenesis of PD (Akundi et al. 2011). Growing evidence also supports a role for aSyn in modulating mitochondrial Ca2+ fluxes. Experiments in neuroblastoma cells overexpressing A53T mutant aSyn demonstrated that aSyn localizes at the mitochondrial membrane and that its accumulation was directly related to an increase of intramitochondrial Ca2+ (Parihar et al. 2008). In line with these findings, another study reported that oligomeric αSyn disrupts mitochondrial function leading to complex I dysfunction, altered membrane potential, disrupted Ca2+ homeostasis and enhanced cytochrome c release (Luth et al. 2014). A possible mechanism recently described supports a role for αSyn in the perturbation of ER-mitochondria associations (Paillusson et al. 2017) (Fig. 2). Indeed, αSyn in PD iPSC-derived neurons and cellular models alters ER–mitochondrial Ca2+ exchange and reduces mitochondrial ATP production by disruption of the VAPB-PTPIP51 tethering proteins. Therefore, the physical link between ER and mitochondria appears to be crucial to regulate Ca2+ accumulation by mitochondria in order to maintain the energetic supply in these neurons and ultimately preserve their survival.
Conclusions and future perspectives
Here, we reviewed the growing evidence suggesting that the preferential demise of SN dopaminergic neurons that characterizes PD results from a combination of cellular insults and the intrinsic susceptibility of DAn arising from their physiology and anatomy. Due to the multifactorial complexity of PD pathogenesis, it is increasingly important to understand and elucidate the cellular mechanisms involved and how they converge, leading to the decline in neuronal function.
Considerable progress in our understanding of cellular pathways in PD pathogenesis came first from the genetic studies that identified genes linked to inherited forms of the disease and risk loci observed associated with the sporadic forms (Labbe and Ross 2014; Nalls et al. 2014). An important goal for those working on the molecular mechanisms of Parkinson’s is to link these genetic findings with cell biology to identify common pathways and themes and, finally, determine shared cellular targets for therapeutic intervention. In our review, we brought together the four themes that we see as central to Parkinson’s: the autophagic/lysosomal pathway, ER stress, intracellular trafficking and calcium signaling. Central to these pathways and at the point of convergence, is lysosomal dysfunction, which we consider a leading current target for therapeutic intervention across Parkinson’s.
The rapid accumulation of new systems-level data requires the development of new approaches to organize and analyze the extensive information on this field. The creation of molecular interaction maps has started to facilitate the detection of integrative pathways and the prioritization of specific targets for further investigation (Kanehisa et al. 2010; Fujita et al. 2014). The emergence of several -omics techniques, including transcriptomics, proteomics and metabolomics, has not only confirmed previously identified pathways associated with DAn degeneration but has also enhanced the ability to identify novel pathways and biomarkers related to PD pathogenesis (Caudle et al. 2010; Ren et al. 2015). Finally, the combination of gene expression data from transcriptomic analysis of patient cells with databases for the Connectivity Map, a technique that analyzes the transcription patterns produced by different chemicals, provides a new opportunity for the identification of potential novel therapeutic compounds for this disorder (Sandor et al. 2017).
References
Abeliovich A, Gitler AD (2016) Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 539(7628):207–216
Ahmed I, Liang Y, Schools S, Dawson VL, Dawson TM, Savitt JM (2012) Development and characterization of a new Parkinson’s disease model resulting from impaired autophagy. J Neurosci 32(46):16503–16509
Akundi RS, Huang Z, Eason J, Pandya JD, Zhi L, Cass WA, Sullivan PG, Bueler H (2011) Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS ONE 6(1):e16038
Alegre-Abarrategui J, Christian H, Lufino MM, Mutihac R, Venda LL, Ansorge O, Wade-Martins R (2009) LRRK2 regulates autophagic activity and localizes to specific membrane microdomains in a novel human genomic reporter cellular model. Hum Mol Genet 18(21):4022–4034
Alegre-Abarrategui J, Wade-Martins R (2009) Parkinson disease, LRRK2 and the endocytic-autophagic pathway. Autophagy 5(8):1208–1210
Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH (2010) Chaperone-mediated autophagy markers in Parkinson disease brains. Arch Neurol 67(12):1464–1472
Bedford C, Sears C, Perez-Carrion M, Piccoli G, Condliffe SB (2016) LRRK2 regulates voltage-gated Calcium Channel function. Front Mol Neurosci 9:35
Beilina A, Rudenko IN, Kaganovich A, Civiero L, Chau H, Kalia SK, Kalia LV, Lobbestael E, Chia R, Ndukwe K, Ding J, Nalls MA, Olszewski M, Hauser DN, Kumaran R, Lozano AM, Baekelandt V, Greene LE, Taymans JM, Greggio E, Cookson MR (2014) Unbiased screen for interactors of leucine-rich repeat kinase 2 supports a common pathway for sporadic and familial Parkinson disease. Proc Natl Acad Sci U S A 111(7):2626–2631
Bendor JT, Logan TP, Edwards RH (2013) The function of alpha-synuclein. Neuron 79(6):1044–1066
Bento CF, Ashkenazi A, Jimenez-Sanchez M, Rubinsztein DC (2016) The Parkinson’s disease-associated genes ATP13A2 and SYT11 regulate autophagy via a common pathway. Nat Commun 7:11803
Biskup S, Moore DJ, Celsi F, Higashi S, West AB, Andrabi SA, Kurkinen K, Yu SW, Savitt JM, Waldvogel HJ, Faull RL, Emson PC, Torp R, Ottersen OP, Dawson TM, Dawson VL (2006) Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann Neurol 60(5):557–569
Bolam JP, Pissadaki EK (2012) Living on the edge with too many mouths to feed: why dopamine neurons die. Mov Disord 27(12):1478–1483
Bonifacino JS, Hurley JH (2008) Retromer. Curr Opin Cell Biol 20(4):427–436
Bouman L, Schlierf A, Lutz AK, Shan J, Deinlein A, Kast J, Galehdar Z, Palmisano V, Patenge N, Berg D, Gasser T, Augustin R, Trumbach D, Irrcher I, Park DS, Wurst W, Kilberg MS, Tatzelt J, Winklhofer KF (2011) Parkin is transcriptionally regulated by ATF4: evidence for an interconnection between mitochondrial stress and ER stress. Cell Death Differ 18(5):769–782
Brini M, Cali T, Ottolini D, Carafoli E (2014) Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci 71(15):2787–2814
Caudle WM, Bammler TK, Lin Y, Pan S, Zhang J (2010) Using ‘omics’ to define pathogenesis and biomarkers of Parkinson’s disease. Expert Rev Neurother 10(6):925–942
Celardo I, Costa AC, Lehmann S, Jones C, Wood N, Mencacci NE, Mallucci GR, Loh SH, Martins LM (2016) Mitofusin-mediated ER stress triggers neurodegeneration in pink1/parkin models of Parkinson’s disease. Cell Death Dis 7(6):e2271
Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ (2007) Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 447(7148):1081–1086
Cherra SJ 3rd, Steer E, Gusdon AM, Kiselyov K, Chu CT (2013) Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am J Pathol 182(2):474–484
Colla E, Coune P, Liu Y, Pletnikova O, Troncoso JC, Iwatsubo T, Schneider BL, Lee MK (2012) Endoplasmic reticulum stress is important for the manifestations of alpha-synucleinopathy in vivo. J Neurosci 32(10):3306–3320
Cooper AA, Gitler AD, Cashikar A, Haynes CM, Hill KJ, Bhullar B, Liu K, Xu K, Strathearn KE, Liu F, Cao S, Caldwell KA, Caldwell GA, Marsischky G, Kolodner RD, Labaer J, Rochet JC, Bonini NM, Lindquist S (2006) Alpha-synuclein blocks ER-Golgi traffic and Rab1 rescues neuron loss in Parkinson’s models. Science 313(5785):324–328
Corti O, Lesage S, Brice A (2011) What genetics tells us about the causes and mechanisms of Parkinson’s disease. Physiol Rev 91(4):1161–1218
Credle JJ, Forcelli PA, Delannoy M, Oaks AW, Permaul E, Berry DL, Duka V, Wills J, Sidhu A (2015) Alpha-Synuclein-mediated inhibition of ATF6 processing into COPII vesicles disrupts UPR signaling in Parkinson’s disease. Neurobiol Dis 76:112–125
Cuervo AM (2010) Chaperone-mediated autophagy: selectivity pays off. Trends Endocrinol Metab 21(3):142–150
Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D (2004) Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 305(5688):1292–1295
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122(Pt 8):1437–1448
Dawson TM, Dawson VL (2010) The role of parkin in familial and sporadic Parkinson’s disease. Mov Disord 25(Suppl 1):S32–S39
Decressac M, Mattsson B, Weikop P, Lundblad M, Jakobsson J, Bjorklund A (2013) TFEB-mediated autophagy rescues midbrain dopamine neurons from alpha-synuclein toxicity. Proc Natl Acad Sci U S A 110(19):E1817–E1826
Dehay B, Ramirez A, Martinez-Vicente M, Perier C, Canron MH, Doudnikoff E, Vital A, Vila M, Klein C, Bezard E (2012) Loss of P-type ATPase ATP13A2/PARK9 function induces general lysosomal deficiency and leads to Parkinson disease neurodegeneration. Proc Natl Acad Sci U S A 109(24):9611–9616
Dodson MW, Zhang T, Jiang C, Chen S, Guo M (2012) Roles of the drosophila LRRK2 homolog in Rab7-dependent lysosomal positioning. Hum Mol Genet 21(6):1350–1363
Dohi E, Tanaka S, Seki T, Miyagi T, Hide I, Takahashi T, Matsumoto M, Sakai N (2012) Hypoxic stress activates chaperone-mediated autophagy and modulates neuronal cell survival. Neurochem Int 60(4):431–442
Dumitriu A, Pacheco CD, Wilk JB, Strathearn KE, Latourelle JC, Goldwurm S, Pezzoli G, Rochet JC, Lindquist S, Myers RH (2011) Cyclin-G-associated kinase modifies alpha-synuclein expression levels and toxicity in Parkinson’s disease: results from the GenePD study. Hum Mol Genet 20(8):1478–1487
Edvardson S, Cinnamon Y, Ta-Shma A, Shaag A, Yim YI, Zenvirt S, Jalas C, Lesage S, Brice A, Taraboulos A, Kaestner KH, Greene LE, Elpeleg O (2012) A deleterious mutation in DNAJC6 encoding the neuronal-specific clathrin-uncoating co-chaperone auxilin, is associated with juvenile parkinsonism. PLoS ONE 7(5):e36458
Fernandes HJ, Hartfield EM, Christian HC, Emmanoulidou E, Zheng Y, Booth H, Bogetofte H, Lang C, Ryan BJ, Sardi SP, Badger J, Vowles J, Evetts S, Tofaris GK, Vekrellis K, Talbot K, Hu MT, James W, Cowley SA, Wade-Martins R (2016) ER stress and Autophagic perturbations lead to elevated extracellular alpha-Synuclein in GBA-N370S Parkinson’s iPSC-derived dopamine neurons. Stem Cell Rep 6(3):342–356
Foehring RC, Zhang XF, Lee JC, Callaway JC (2009) Endogenous calcium buffering capacity of substantia nigral dopamine neurons. J Neurophysiol 102(4):2326–2333
Follett J, Norwood SJ, Hamilton NA, Mohan M, Kovtun O, Tay S, Zhe Y, Wood SA, Mellick GD, Silburn PA, Collins BM, Bugarcic A, Teasdale RD (2014) The Vps35 D620N mutation linked to Parkinson’s disease disrupts the cargo sorting function of retromer. Traffic 15(2):230–244
Friedman LG, Lachenmayer ML, Wang J, He L, Poulose SM, Komatsu M, Holstein GR, Yue Z (2012) Disrupted autophagy leads to dopaminergic axon and dendrite degeneration and promotes presynaptic accumulation of alpha-synuclein and LRRK2 in the brain. J Neurosci 32(22):7585–7593
Fujita KA, Ostaszewski M, Matsuoka Y, Ghosh S, Glaab E, Trefois C, Crespo I, Perumal TM, Jurkowski W, Antony PM, Diederich N, Buttini M, Kodama A, Satagopam VP, Eifes S, Del Sol A, Schneider R, Kitano H, Balling R (2014) Integrating pathways of Parkinson’s disease in a molecular interaction map. Mol Neurobiol 49(1):88–102
Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY (2009) PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell 33(5):627–638
Gitler AD, Bevis BJ, Shorter J, Strathearn KE, Hamamichi S, Su LJ, Caldwell KA, Caldwell GA, Rochet JC, McCaffery JM, Barlowe C, Lindquist S (2008) The Parkinson’s disease protein alpha-synuclein disrupts cellular Rab homeostasis. Proc Natl Acad Sci U S A 105(1):145–150
Gomez-Suaga P, Rivero-Rios P, Fdez E, Blanca Ramirez M, Ferrer I, Aiastui A, Lopez De Munain A, Hilfiker S (2014) LRRK2 delays degradative receptor trafficking by impeding late endosomal budding through decreasing Rab7 activity. Hum Mol Genet 23(25):6779–6796
Grace AA, Bunney BS (1983) Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience 10(2):301–315
Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S (2014) Alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci 34(1):249–259
Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ (2010) Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature 468(7324):696–700
Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N (2006) Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441(7095):885–889
Heeman B, Van den Haute C, Aelvoet SA, Valsecchi F, Rodenburg RJ, Reumers V, Debyser Z, Callewaert G, Koopman WJ, Willems PH, Baekelandt V (2011) Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci 124(Pt 7):1115–1125
Henry AG, Aghamohammadzadeh S, Samaroo H, Chen Y, Mou K, Needle E, Hirst WD (2015) Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum Mol Genet 24(21):6013–6028
Hetz C (2012) The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 13(2):89–102
Higashi S, Moore DJ, Yamamoto R, Minegishi M, Sato K, Togo T, Katsuse O, Uchikado H, Furukawa Y, Hino H, Kosaka K, Emson PC, Wada K, Dawson VL, Dawson TM, Arai H, Iseki E (2009) Abnormal localization of leucine-rich repeat kinase 2 to the endosomal-lysosomal compartment in lewy body disease. J Neuropathol Exp Neurol 68(9):994–1005
Hoozemans JJ, van Haastert ES, Eikelenboom P, de Vos RA, Rozemuller JM, Scheper W (2007) Activation of the unfolded protein response in Parkinson’s disease. Biochem Biophys Res Commun 354(3):707–711
Horowitz MP, Greenamyre JT (2010) Gene-environment interactions in Parkinson’s disease: the importance of animal modeling. Clin Pharmacol Ther 88(4):467–474
Hunn BH, Cragg SJ, Bolam JP, Spillantini MG, Wade-Martins R (2015) Impaired intracellular trafficking defines early Parkinson’s disease. Trends Neurosci 38(3):178–188
Hurley MJ, Dexter DT (2012) Voltage-gated calcium channels and Parkinson’s disease. Pharmacol Ther 133(3):324–333
Imai Y, Soda M, Inoue H, Hattori N, Mizuno Y, Takahashi R (2001) An unfolded putative transmembrane polypeptide, which can lead to endoplasmic reticulum stress, is a substrate of Parkin. Cell 105(7):891–902
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79(4):368–376
Jeong JK, Moon MH, Lee YJ, Seol JW, Park SY (2013) Autophagy induced by the class III histone deacetylase Sirt1 prevents prion peptide neurotoxicity. Neurobiol Aging 34(1):146–156
Jo E, McLaurin J, Yip CM, St George-Hyslop P, Fraser PE (2000) Alpha-Synuclein membrane interactions and lipid specificity. J Biol Chem 275(44):34328–34334
Kanehisa M, Limviphuvadh V, Tanabe M (2010) Knowledge-based analysis of protein interaction networks in neurodegenerative diseases . In: Alzate O (ed)Neuroproteomics. CRC, Boca Raton, pp 147–162
Klionsky DJ (2005) The molecular machinery of autophagy: unanswered questions. J Cell Sci 118(Pt 1):7–18
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884
Kon M, Cuervo AM (2010) Chaperone-mediated autophagy in health and disease. FEBS Lett 584(7):1399–1404
Kunz JB, Schwarz H, Mayer A (2004) Determination of four sequential stages during microautophagy in vitro. J Biol Chem 279(11):9987–9996
Labbe C, Ross OA (2014) Association studies of sporadic Parkinson’s disease in the genomic era. Curr Genomics 15(1):2–10
Laguna A, Schintu N, Nobre A, Alvarsson A, Volakakis N, Jacobsen JK, Gomez-Galan M, Sopova E, Joodmardi E, Yoshitake T, Deng Q, Kehr J, Ericson J, Svenningsson P, Shupliakov O, Perlmann T (2015) Dopaminergic control of autophagic-lysosomal function implicates Lmx1b in Parkinson’s disease. Nat Neurosci 18(6):826–835
Lee AS (2005) The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods 35(4):373–381
Lee DW, Wu X, Eisenberg E, Greene LE (2006) Recruitment dynamics of GAK and auxilin to clathrin-coated pits during endocytosis. J Cell Sci 119(Pt 17):3502–3512
Lin X, Parisiadou L, Gu XL, Wang L, Shim H, Sun L, Xie C, Long CX, Yang WJ, Ding J, Chen ZZ, Gallant PE, Tao-Cheng JH, Rudow G, Troncoso JC, Liu Z, Li Z, Cai H (2009) Leucine-rich repeat kinase 2 regulates the progression of neuropathology induced by Parkinson’s-disease-related mutant alpha-synuclein. Neuron 64(6):807–827
Lubbe S, Morris HR (2014) Recent advances in Parkinson’s disease genetics. J Neurol 261(2):259–266
Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ (2014) Soluble, prefibrillar alpha-synuclein oligomers promote complex I-dependent, Ca2+−induced mitochondrial dysfunction. J Biol Chem 289(31):21490–21507
MacLeod DA, Rhinn H, Kuwahara T, Zolin A, Di Paolo G, McCabe BD, Marder KS, Honig LS, Clark LN, Small SA, Abeliovich A (2013) RAB7L1 interacts with LRRK2 to modify intraneuronal protein sorting and Parkinson’s disease risk. Neuron 77(3):425–439
Magalhaes J, Gegg ME, Migdalska-Richards A, Doherty MK, Whitfield PD, Schapira AH (2016) Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: relevance to Parkinson disease. Hum Mol Genet 25(16):3432–3445
Manzoni C, Mamais A, Dihanich S, McGoldrick P, Devine MJ, Zerle J, Kara E, Taanman JW, Healy DG, Marti-Masso JF, Schapira AH, Plun-Favreau H, Tooze S, Hardy J, Bandopadhyay R, Lewis PA (2013) Pathogenic Parkinson’s disease mutations across the functional domains of LRRK2 alter the autophagic/lysosomal response to starvation. Biochem Biophys Res Commun 441(4):862–866
Marongiu R, Spencer B, Crews L, Adame A, Patrick C, Trejo M, Dallapiccola B, Valente EM, Masliah E (2009) Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J Neurochem 108(6):1561–1574
Martinez-Vicente M, Talloczy Z, Kaushik S, Massey AC, Mazzulli J, Mosharov EV, Hodara R, Fredenburg R, Wu DC, Follenzi A, Dauer W, Przedborski S, Ischiropoulos H, Lansbury PT, Sulzer D, Cuervo AM (2008) Dopamine-modified alpha-synuclein blocks chaperone-mediated autophagy. J Clin Invest 118(2):777–788
Matus S, Lisbona F, Torres M, Leon C, Thielen P, Hetz C (2008) The stress rheostat: an interplay between the unfolded protein response (UPR) and autophagy in neurodegeneration. Curr Mol Med 8(3):157–172
Mazzulli JR, Xu YH, Sun Y, Knight AL, McLean PJ, Caldwell GA, Sidransky E, Grabowski GA, Krainc D (2011) Gaucher disease glucocerebrosidase and alpha-synuclein form a bidirectional pathogenic loop in synucleinopathies. Cell 146(1):37–52
Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D (2016) Alpha-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci U S A 113(7):1931–1936
McCaffrey K, Braakman I (2016) Protein quality control at the endoplasmic reticulum. Essays Biochem 60(2):227–235
McGough IJ, Steinberg F, Jia D, Barbuti PA, McMillan KJ, Heesom KJ, Whone AL, Caldwell MA, Billadeau DD, Rosen MK, Cullen PJ (2014) Retromer binding to FAM21 and the WASH complex is perturbed by the Parkinson disease-linked VPS35(D620N) mutation. Curr Biol 24(14):1670–1676
Melachroinou K, Xilouri M, Emmanouilidou E, Masgrau R, Papazafiri P, Stefanis L, Vekrellis K (2013) Deregulation of calcium homeostasis mediates secreted alpha-synuclein-induced neurotoxicity. Neurobiol Aging 34(12):2853–2865
Murphy KE, Gysbers AM, Abbott SK, Spiro AS, Furuta A, Cooper A, Garner B, Kabuta T, Halliday GM (2015) Lysosomal-associated membrane protein 2 isoforms are differentially affected in early Parkinson’s disease. Mov Disord 30(12):1639–1647
Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, Sidransky E, Cooper A, Garner B, Halliday GM (2014) Reduced glucocerebrosidase is associated with increased alpha-synuclein in sporadic Parkinson’s disease. Brain 137(Pt 3):834–848
Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB (2014) Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat Genet 46(9):989–993
Nikoletopoulou V, Papandreou ME, Tavernarakis N (2015) Autophagy in the physiology and pathology of the central nervous system. Cell Death Differ 22(3):398–407
Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K (2006) Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol 26(24):9220–9231
Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, Raya A, Sulzer D, Cuervo AM (2013) Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 16(4):394–406
Ottolini D, Cali T, Negro A, Brini M (2013) The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum Mol Genet 22(11):2152–2168
Paillusson S, Gomez-Suaga P, Stoica R, Little D, Gissen P, Devine MJ, Noble W, Hanger DP, Miller CC (2017) Alpha-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Neuropathol Acta
Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P (2008) Mitochondrial association of alpha-synuclein causes oxidative stress. Cell Mol Life Sci 65(7–8):1272–1284
Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C (2006) Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet 38(10):1184–1191
Ramonet D, Daher JP, Lin BM, Stafa K, Kim J, Banerjee R, Westerlund M, Pletnikova O, Glauser L, Yang L, Liu Y, Swing DA, Beal MF, Troncoso JC, McCaffery JM, Jenkins NA, Copeland NG, Galter D, Thomas B, Lee MK, Dawson TM, Dawson VL, Moore DJ (2011) Dopaminergic neuronal loss, reduced neurite complexity and autophagic abnormalities in transgenic mice expressing G2019S mutant LRRK2. PLoS ONE 6(4):e18568
Rashid HO, Yadav RK, Kim HR, Chae HJ (2015) ER stress: Autophagy induction, inhibition and selection. Autophagy 11(11):1956–1977
Ren R, Sun Y, Zhao X, Pu X (2015) Recent advances in biomarkers for Parkinson’s disease focusing on biochemicals, omics and neuroimaging. Clin Chem Lab Med 53(10):1495–1506
Reznichenko L, Cheng Q, Nizar K, Gratiy SL, Saisan PA, Rockenstein EM, Gonzalez T, Patrick C, Spencer B, Desplats P, Dale AM, Devor A, Masliah E (2012) In vivo alterations in calcium buffering capacity in transgenic mouse model of synucleinopathy. J Neurosci 32(29):9992–9998
Rodriguez-Muela N, Boya P (2012) Axonal damage, autophagy and neuronal survival. Autophagy 8(2):286–288
Ron I, Horowitz M (2005) ER retention and degradation as the molecular basis underlying Gaucher disease heterogeneity. Hum Mol Genet 14(16):2387–2398
Sanchez-Danes A, Richaud-Patin Y, Carballo-Carbajal I, Jimenez-Delgado S, Caig C, Mora S, Di Guglielmo C, Ezquerra M, Patel B, Giralt A, Canals JM, Memo M, Alberch J, Lopez-Barneo J, Vila M, Cuervo AM, Tolosa E, Consiglio A, Raya A (2012) Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med 4(5):380–395
Sandor C, Robertson P, Lang C, Heger A, Booth H, Vowles J, Witty L, Bowden R, Hu M, Cowley SA, Wade-Martins R, Webber C (2017) Transcriptomic profiling of purified patient-derived dopamine neurons identifies convergent perturbations and therapeutics for Parkinson’s disease. Hum Mol Genet 26(3):552–566
Schondorf DC, Aureli M, McAllister FE, Hindley CJ, Mayer F, Schmid B, Sardi SP, Valsecchi M, Hoffmann S, Schwarz LK, Hedrich U, Berg D, Shihabuddin LS, Hu J, Pruszak J, Gygi SP, Sonnino S, Gasser T, Deleidi M (2014) iPSC-derived neurons from GBA1-associated Parkinson’s disease patients show autophagic defects and impaired calcium homeostasis. Nat Commun 5:4028
Schwab AJ, Ebert AD (2015) Neurite aggregation and calcium dysfunction in iPSC-derived sensory neurons with Parkinson’s disease-related LRRK2 G2019S mutation. Stem Cell Rep 5(6):1039–1052
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361(17):1651–1661
Smith WW, Jiang H, Pei Z, Tanaka Y, Morita H, Sawa A, Dawson VL, Dawson TM, Ross CA (2005) Endoplasmic reticulum stress and mitochondrial cell death pathways mediate A53T mutant alpha-synuclein-induced toxicity. Hum Mol Genet 14(24):3801–3811
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388(6645):839–840
Steger M, Tonelli F, Ito G, Davies P, Trost M, Vetter M, Wachter S, Lorentzen E, Duddy G, Wilson S, Baptista MA, Fiske BK, Fell MJ, Morrow JA, Reith AD, Alessi DR, Mann M (2016) Phosphoproteomics reveals that Parkinson’s disease kinase LRRK2 regulates a subset of Rab GTPases. eLife 5
Surmeier DJ (2007) Calcium, ageing, and neuronal vulnerability in Parkinson’s disease. Lancet Neurol 6(10):933–938
Tang FL, Erion JR, Tian Y, Liu W, Yin DM, Ye J, Tang B, Mei L, Xiong WC (2015) VPS35 in dopamine neurons is required for Endosome-to-Golgi retrieval of Lamp2a, a receptor of chaperone-mediated Autophagy that is critical for alpha-Synuclein degradation and prevention of pathogenesis of Parkinson’s disease. J Neurosci 35(29):10613–10628
Tanik SA, Schultheiss CE, Volpicelli-Daley LA, Brunden KR, Lee VM (2013) Lewy body-like alpha-synuclein aggregates resist degradation and impair macroautophagy. J Biol Chem 288(21):15194–15210
Thayanidhi N, Helm JR, Nycz DC, Bentley M, Liang Y, Hay JC (2010) Alpha-synuclein delays endoplasmic reticulum (ER)-to-Golgi transport in mammalian cells by antagonizing ER/Golgi SNAREs. Mol Biol Cell 21(11):1850–1863
Tokarev AA, Alfonso A, Segev N (2009) Overview of intracellular compartments and trafficking pathways. In: Segev N (ed)Trafficking inside cells: pathways, mechanisms and regulation. Springer, New York, pp 3–14
Tsika E, Glauser L, Moser R, Fiser A, Daniel G, Sheerin UM, Lees A, Troncoso JC, Lewis PA, Bandopadhyay R, Schneider BL, Moore DJ (2014) Parkinson’s disease-linked mutations in VPS35 induce dopaminergic neurodegeneration. Hum Mol Genet 23(17):4621–4638
Usenovic M, Knight AL, Ray A, Wong V, Brown KR, Caldwell GA, Caldwell KA, Stagljar I, Krainc D (2012a) Identification of novel ATP13A2 interactors and their role in alpha-synuclein misfolding and toxicity. Hum Mol Genet 21(17):3785–3794
Usenovic M, Tresse E, Mazzulli JR, Taylor JP, Krainc D (2012b) Deficiency of ATP13A2 leads to lysosomal dysfunction, alpha-synuclein accumulation, and neurotoxicity. J Neurosci 32(12):4240–4246
Uversky VN (2007) Neuropathology, biochemistry, and biophysics of alpha-synuclein aggregation. J Neurochem 103(1):17–37
Vilarino-Guell C, Rajput A, Milnerwood AJ, Shah B, Szu-Tu C, Trinh J, Yu I, Encarnacion M, Munsie LN, Tapia L, Gustavsson EK, Chou P, Tatarnikov I, Evans DM, Pishotta FT, Volta M, Beccano-Kelly D, Thompson C, Lin MK, Sherman HE, Han HJ, Guenther BL, Wasserman WW, Bernard V, Ross CJ, Appel-Cresswell S, Stoessl AJ, Robinson CA, Dickson DW, Ross OA, Wszolek ZK, Aasly JO, Wu RM, Hentati F, Gibson RA, McPherson PS, Girard M, Rajput M, Rajput AH, Farrer MJ (2014) DNAJC13 mutations in Parkinson disease. Hum Mol Genet 23(7):1794–1801
Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283(35):23542–23556
Volpicelli-Daley LA, Gamble KL, Schultheiss CE, Riddle DM, West AB, Lee VM (2014) Formation of alpha-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell 25(25):4010–4023
Wallings R, Manzoni C, Bandopadhyay R (2015) Cellular processes associated with LRRK2 function and dysfunction. FEBS J 282(15):2806–2826
Wang X, Huang T, Bu G, Xu H (2014) Dysregulation of protein trafficking in neurodegeneration. Mol Neurodegener 9:31
Weinreb PH, Zhen W, Poon AW, Conway KA, Lansbury PT Jr (1996) NACP, a protein implicated in Alzheimer’s disease and learning, is natively unfolded. Biochemistry 35(43):13709–13715
Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O’Kane CJ, Rubinsztein DC (2010) Alpha-Synuclein impairs macroautophagy: implications for Parkinson’s disease. J Cell Biol 190(6):1023–1037
Xu W, Lipscombe D (2001) Neuronal ca(V)1.3alpha(1) L-type channels activate at relatively hyperpolarized membrane potentials and are incompletely inhibited by dihydropyridines. J Neurosci 21(16):5944–5951
Yan CH, Levesque M, Claxton S, Johnson RL, Ang SL (2011) Lmx1a and lmx1b function cooperatively to regulate proliferation, specification, and differentiation of midbrain dopaminergic progenitors. J Neurosci 31(35):12413–12425
Zavodszky E, Seaman MN, Moreau K, Jimenez-Sanchez M, Breusegem SY, Harbour ME, Rubinsztein DC (2014) Mutation in VPS35 associated with Parkinson’s disease impairs WASH complex association and inhibits autophagy. Nat Commun 5:3828
Zimprich A, Biskup S, Leitner P, Lichtner P, Farrer M, Lincoln S, Kachergus J, Hulihan M, Uitti RJ, Calne DB, Stoessl AJ, Pfeiffer RF, Patenge N, Carbajal IC, Vieregge P, Asmus F, Muller-Myhsok B, Dickson DW, Meitinger T, Strom TM, Wszolek ZK, Gasser T (2004) Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron 44(4):601–607
Acknowledgements
Work in the Wade-Martins Laboratory is supported by the Monument Trust Discovery Award from Parkinson’s UK (Grant J-1403). M.C. is supported by a collaborative grant from Mission Therapeutics, Cambridge UK.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Cherubini, M., Wade-Martins, R. Convergent pathways in Parkinson’s disease. Cell Tissue Res 373, 79–90 (2018). https://doi.org/10.1007/s00441-017-2700-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-017-2700-2