Abstract
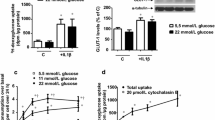
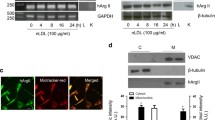
High glucose induces vascular smooth muscle cell (SMC) dysfunction by generating oxidative stress attributable, in part, to the up-regulated NADPH oxidases (Nox). We have attempted to elucidate the high-glucose-generated molecular signals that mediate this effect and hypothesize that products of high-glucose-induced lipid peroxidation regulate Nox by activating peroxisome proliferator-activated receptors (PPARs). Human aortic SMCs were exposed to glucose (5.5–25 mM) or 4-hydroxynonenal (1–25 μM, 4-HNE). Lucigenin assay, real-time polymerase chain reaction, western blot, and promoter analyses were employed to investigate Nox. We found that high glucose generated an increase in Nox activity and expression. It also promoted oxidative stress that consequently induced lipid peroxidation, which resulted in the production of 4-HNE. Pharmacological inhibition of Nox activity significantly reduced the formation of high-glucose-induced 4-HNE. Exposure of SMCs to non-cytotoxic concentrations (1–10 μM) of 4-HNE alone mimicked the effect of high glucose incubation, whereas scavenging of 4-HNE by N-acetyl L-cysteine completely abolished both the effects of high glucose and 4-HNE. The latter exerted its effect by activating PPARα and PPARβ/δ, but not PPARγ, as assessed pharmacologically by the inhibitory effect of selective antagonists and following the silencing of the expression of these receptors. These new data indicate that 4-HNE, generated following Nox activation, functions as an endogenous activator of PPARα and PPARβ/δ. The newly discovered “lipid peroxidation products–PPARs–Nox axis” represents a novel mechanism of Nox regulation and an additional therapeutic target for oxidative stress in diabetes.





Similar content being viewed by others
References
Calkin AC, Cooper ME, Jandeleit-Dahm KA, Allen TJ (2006) Gemfibrozil decreases atherosclerosis in experimental diabetes in association with a reduction in oxidative stress and inflammation. Diabetologia 49:766–774
Cohen G, Riahi Y, Shamni O, Guichardant M, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S (2011) Role of lipid peroxidation and PPAR-δ in amplifying glucose-stimulated insulin secretion. Diabetes 60:2830–2842
Cohen G, Riahi Y, Sasson S (2012) Free radicals and metabolic disorders. In: Chatgilialoglu C, Studer A (eds) Handbook of radicals in chemistry and biology. Wiley, Chichester, pp 1679–1700
Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, Kaiser N, Sasson S (2013) Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med 65:978–987
Coleman JD, Prabhu KS, Thompson JT, Reddy PS, Peters JM, Peterson BR, Reddy CC, Vanden Heuvel JP (2007) The oxidative stress mediator 4-hydroxynonenal is an intracellular agonist of the nuclear receptor peroxisome proliferator-activated receptor-beta/delta (PPARbeta/delta). Free Radic Biol Med 42:1155–1164
Csányi G, Taylor WR, Pagano PJ (2009) NOX and inflammation in the vascular adventitia. Free Radic Biol Med 47:1254–1266
Das SK, Chakrabarti R (2006) Role of PPAR in cardiovascular diseases. Recent Pat Cardiovasc Drug Discov 1:193–209
Ding H, Hashem M, Triggle C (2007) Increased oxidative stress in the streptozotocin-induced diabetic apoE-deficient mouse: changes in expression of NADPH oxidase subunits and eNOS. Eur J Pharmacol 561:121–128
Ellmark SH, Dusting GJ, Fui MN, Guzzo-Pernell N, Drummond GR (2005) The contribution of Nox4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovasc Res 65:495–504
Forman BM, Chen J, Evans RM (1997) Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors alpha and delta. Proc Natl Acad Sci U S A 94:4312–4317
Gao L, Mann GE (2009) Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signaling. Cardiovasc Res 82:9–20
Gervois P, Fruchart JC, Staels B (2004) Inflammation, dyslipidaemia, diabetes and PPARs: pharmacological interest of dual PPARalpha and PPARgamma agonists. Int J Clin Pract Suppl 143:22–29
Gizard F, Amant C, Barbier O, Bellosta S, Robillard R, Percevault F, Sevestre H, Krimpenfort P, Corsini A, Rochette J, Glineur C, Fruchart JC, Torpier G, Staels B (2005) PPAR alpha inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4a. J Clin Invest 115:3228–3238
Gray SP, Di Marco E, Okabe J, Szyndralewiez C, Heitz F, Montezano AC, de Haan JB, Koulis C, El-Osta A, Andrews KL, Chin-Dusting JP, Touyz RM, Wingler K, Cooper ME, Schmidt HH, Jandeleit-Dahm KA (2013) NADPH oxidase 1 plays a key role in diabetes mellitus-accelerated atherosclerosis. Circulation 127:1888–1902
Gross B, Staels B (2007) PPAR agonists: multimodal drugs for the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab 21:687–710
Heltianu C, Guja C, Manea SA (2011) Genetic determinants of microvascular complications in type 1 diabetes. In: Wagner D (ed) Type 1 diabetes complications. InTech Open Access, Rijeka, pp 3–28
Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51:211–217
Kahles T, Brandes RP (2012) NADPH oxidases as therapeutic targets in ischemic stroke. Cell Mol Life Sci 69:2345–2363
Kimura H, Liu S, Yamada S, Uchida K, Matsumoto K, Mukaida M, Yoshida K (2005) Rapid increase in serum lipid peroxide 4-hydroxynonenal (HNE) through monocyte NADPH oxidase in early endo-toxemia. Free Radic Res 39:845–851
Krause KH, Lambeth D, Krönke M (2012) NOX enzymes as drug targets. Cell Mol Life Sci 69:2279–2282
Lalloyer F, Wouters K, Baron M, Caron S, Vallez E, Vanhoutte J, Baugé E, Shiri-Sverdlov R, Hofker M, Staels B, Tailleux A (2011) Peroxisome proliferator-activated receptor-alpha gene level differently affects lipid metabolism and inflammation in apolipoprotein E2 knock-in mice. Arterioscler Thromb Vasc Biol 31:1573–1579
Li WG, Stoll LL, Rice JB, Xu SP, Miller FJ Jr, Chatterjee P, Hu L, Oberley LW, Spector AA, Weintraub NL (2003) Activation of NAD(P)H oxidase by lipid hydroperoxides: mechanism of oxidant-mediated smooth muscle cytotoxicity. Free Radic Biol Med 34:937–946
Manea A (2010) NADPH oxidase-derived reactive oxygen species: involvement in vascular physiology and pathology. Cell Tissue Res 342:325–339
Manea A (2011) Vascular biology of reactive oxygen species and NADPH oxidases: role in atherogenesis. In: Paryhasarathy S (ed) Atherogenesis. InTech Open Access, Rijeka, 425–446
Manea A, Simionescu M (2012) Nox enzymes and oxidative stress in atherosclerosis. Front Biosci (Schol Ed) 4:651–670
Manea A, Manea SA, Gafencu AV, Raicu M, Simionescu M (2008) AP-1-dependent transcriptional regulation of NADPH oxidase in human aortic smooth muscle cells: role of p22phox subunit. Arterioscler Thromb Vasc Biol 28:878–885
Manea A, Tanase LI, Raicu M, Simionescu M (2010a) Jak/STAT signaling pathway regulates Nox1 and Nox4-based NADPH oxidase in human aortic smooth muscle cells. Arterioscler Thromb Vasc Biol 30:105–112
Manea A, Tanase LI, Raicu M, Simionescu M (2010b) Transcriptional regulation of NADPH oxidase isoforms, Nox1 and Nox4, by nuclear factor-kappaB in human aortic smooth muscle cells. Biochem Biophys Res Commun 396:901–907
Manea A, Manea SA, Florea IC, Luca CM, Raicu M (2012) Positive regulation of NADPH oxidase 5 by proinflammatory-related mechanisms in human aortic smooth muscle cells. Free Radic Biol Med 52:1497–1507
Manea SA, Todirita A, Manea A (2013) High glucose-induced increased expression of endothelin-1 in human endothelial cells is mediated by activated CCAAT/enhancer-binding proteins. PLoS One 8:e84170
Manea SA, Todirita A, Raicu M, Manea A (2014) C/EBP transcription factors regulate NADPH oxidase in human aortic smooth muscle cells. J Cell Mol Med 18:1467–1477
Okazaki M, Iwasaki Y, Nishiyama M, Taguchi T, Tsugita M, Nakayama S, Sevestre H, Krimpenfort P, Corsini A, Rochette J, Glineur C, Fruchart JC, Torpier G, Staels B (2010) PPARbeta/delta regulates the human SIRT1 gene transcription via Sp1. Endocr J 57:403–413
Plutzky J (2011) The PPAR-RXR transcriptional complex in the vasculature: energy in the balance. Circ Res 108:1002–1016
Quintela AM, Jiménez R, Gómez-Guzmán M, Zarzuelo MJ, Galindo P, Sánchez M, Vargas F, Cogolludo A, Tamargo J, Pérez-Vizcaíno F, Duarte J (2012) Activation of peroxisome proliferator-activated receptor-β/-δ (PPARβ/δ) prevents endothelial dysfunction in type 1 diabetic rats. Free Radic Biol Med 53:730–741
Riahi Y, Cohen G, Shamni O, Sasson S (2010a) Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab 299:E879–E886
Riahi Y, Sin-Malia Y, Cohen G, Alpert E, Gruzman A, Eckel J, Staels B, Guichardant M, Sasson S (2010b) The natural protective mechanism against hyperglycemia in vascular endothelial cells: roles of the lipid peroxidation product 4-hydroxydodecadienal and peroxisome proliferator-activated receptor delta. Diabetes 59:808–818
Ricote M, Glass CK (2007) PPARs and molecular mechanisms of transrepression. Biochim Biophys Acta 1771:926–993
Russo GL (2009) Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem Pharmacol 77:937–946
Schlüter T, Steinbach AC, Steffen A, Rettig R, Grisk O (2008) Apocynin-induced vasodilation involves Rho kinase inhibition but not NADPH oxidase inhibition. Cardiovasc Res 80:271–279
Sedeek M, Callera G, Montezano A, Gutsol AF, Heitz F, Szyndralewiez C, Page P, Kennedy CR, Burns KD, Touyz RM, Hébert RL (2012a) Critical role of Nox4-based NADPH oxidase in glucose-induced oxidative stress in the kidney: implications in type 2 diabetic nephropathy. Am J Physiol Ren Physiol 299:F1348–F1358
Sedeek M, Montezano AC, Hebert RL, Gray SP, Di Marco E, Jha JC, Cooper ME, Jandeleit-Dahm K, Schiffrin EL, Wilkinson-Berka JL, Touyz RM (2012b) Oxidative stress, Nox isoforms and complications of diabetes-potential targets for novel therapies. J Cardiovasc Transl Res 5:509–518
Sharifpanah F, Wartenberg M, Hannig M, Piper HM, Sauer H (2008) Peroxisome proliferator-activated receptor alpha agonists enhance cardiomyogenesis of mouse ES cells by utilization of a reactive oxygen species-dependent mechanism. Stem Cells 26:64–71
Staels B (2007) PPAR agonists and the metabolic syndrome. Therapie 62:319–332
Staels B, Fruchart JC (2005) Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes 54:2460–2470
Stefanska J, Pawliczak R (2008) Apocynin: molecular aptitudes. Mediat Inflamm 2008:106507
Teissier E, Nohara A, Chinetti G, Paumelle R, Cariou B, Fruchart JC, Brandes RP, Shah A, Staels B (2004) Peroxisome proliferator-activated receptor alpha induces NADPH oxidase activity in macrophages, leading to the generation of LDL with PPAR-alpha activation properties. Circ Res 95:1174–1182
Tîrziu D, Jinga VV, Serban G, Simionescu M (1999) The effects of low density lipoproteins modified by incubation with chondroitin 6-sulfate on human aortic smooth muscle cells. Atherosclerosis 147:155–166
Tordjman KM, Semenkovich CF, Coleman T, Yudovich R, Bak S, Osher E, Vechoropoulos M, Stern N (2007) Absence of peroxisome proliferator-activated receptor-alpha abolishes hypertension and attenuates atherosclerosis in the Tsukuba hypertensive mouse. Hypertension 50:945–951
Touyz RM, Chen X, Tabet F, Yao G, He G, Quinn MT, Pagano PJ, Schiffrin EL (2002) Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: regulation by angiotensin II. Circ Res 90:1205–1213
Ungvari Z, Csiszar A, Edwards JG, Kaminski PM, Wolin MS, Kaley G, Koller A (2003) Increased superoxide production in coronary arteries in hyperhomocysteinemia: role of tumor necrosis factor-alpha, NAD(P)H oxidase, and inducible nitric oxide synthase. Arterioscler Thromb Vasc Biol 23:418–424
Yagil C, Yagil Y (2007) Peroxisome proliferator-activated receptor alpha: friend or foe? Hypertension 50:847–850
Yun MR, Park HM, Seo KW, Lee SJ, Im DS, Kim CD (2010) 5-Lipoxygenase plays an essential role in 4-HNE-enhanced ROS production in murine macrophages via activation of NADPH oxidase. Free Radic Res 44:742–750
Zalba G, San José G, Beaumont FJ, Fortuño MA, Fortuño A, Díez J (2001) Polymorphisms and promoter overactivity of the p22(phox) gene in vascular smooth muscle cells from spontaneously hypertensive rats. Circ Res 88:217–222
Acknowledgments
We acknowledge the skillful assistance of Floarea Georgescu, Camelia Matei, and Constanta Stan.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by the European Foundation for the Study of Diabetes – The New Horizons Grant to A. Manea in collaboration with S. Sasson, Romanian National Authority for Scientific Research (CNCS-UEFISCDI, project numbers PN-II-ID-PCE-2011-3-0548, PN-II-RU-TE-2011-3-0142, PNII-TE 65/2010), and COST action BM1203/EU-ROS. Simona A. Manea acknowledges the support of the strategic grant POSDRU/159/1.5/S/133391 financed by the European Social Found within the Sectorial Operational Program Human Resources Development 2007–2013. S. Sasson is the Adolf D. and Horty Storch Chair in Pharmaceutical Sciences, at the Faculty of Medicine, The Hebrew University of Jerusalem, Israel. He is affiliated with the David R. Bloom Center for Pharmacy and the Dr. Adolf and Klara Brettler Center for Research of Molecular Pharmacology and Therapeutics in the Hebrew University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 741 kb)
Rights and permissions
About this article
Cite this article
Manea, A., Manea, SA., Todirita, A. et al. High-glucose-increased expression and activation of NADPH oxidase in human vascular smooth muscle cells is mediated by 4-hydroxynonenal-activated PPARα and PPARβ/δ. Cell Tissue Res 361, 593–604 (2015). https://doi.org/10.1007/s00441-015-2120-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-015-2120-0