Abstract
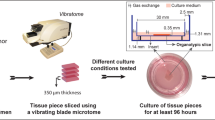
Rapamycin is a selective inhibitor of the mammalian target of rapamycin (mTOR), a regulator kinase that integrates growth factors signaling via the phosphoinositide-3-kinase pathway and that has emerged as a novel therapeutic modality in breast cancer (BC). We propose a pre-clinical “ex-vivo” personalized organotypic culture of BC that preserves the microenvironment to evaluate rapamycin-mediated gene expression changes. Freshly excised ductal invasive BC slices, 400 μm thick (n=30), were cultured in the presence or absence (control) of rapamycin (20 nM) for 24 h. Some slices were formalin-fixed for immunohistochemical determinations and some were processed for microarray analysis. Control slices in culture retained their tissue morphology and tissue viability (detected by BrdU uptake). The percentage of proliferating cells (assessed by Ki67) did not change up to 24 h of treatment. Immunohistochemical evaluation of p-AKT, p-mTOR, p-4EBP1 and p-S6K1 indicated that AKT/mTOR pathway activation was maintained during cultivation. For microarray analysis, slices were divided into two groups, according to the presence/absence of epidermal growth factor receptor-type 2 and analyzed separately. Limited overlap was seen among differentially expressed genes after treatment (P<0.01) in both groups suggesting different responses to rapamycin between these BC subtypes. Ontology analysis indicated that genes involved in biosynthetic processes were commonly reduced by rapamycin. Our network analysis suggested that concerted expression of these genes might distinguish controls from treated slices. Thus, breast carcinoma slices constitute a suitable physiological tool to evaluate the short-term effects of rapamycin on the gene profile of individual BC samples.






Similar content being viewed by others
References
Akcakanat A, Zhang L, Tsavachidis S, Meric-Bernstam F (2009) The rapamycin-regulated gene expression signature determines prognosis for breast cancer. Mol Cancer 8:75
Andre F, Nahta R, Conforti R, Boulet T, Aziz M, Yuan LX et al (2008) Expression patterns and predictive value of phosphorylated AKT in early-stage breast cancer. Ann Oncol 19:315–320
Barabási AL, Oltvai ZN (2004) Network biology: understanding the cell’s functional organization. Nat Rev Genet 5:101–113
Barbosa EM, Nonogaki S, Katayama ML, Folgueira MA, Alves VF, Brentani MM (2004) Vitamin D3 modulation of plasminogen activator inhibitor type-1 in human breast carcinomas under organ culture. Virchows Arch 444:175–182
Bläuer M, Tammela TL, Ylikomi T (2008) A novel tissue-slice culture model for non-malignant human prostate. Cell Tissue Res 332:489–498
Bollig-Fischer A, Dewey TG, Ethier SP (2011) Oncogene activation induces metabolic transformation resulting in insulin-independence in human breast cancer cells. PLoS One 6:e17959
Bose S, Chandran S, Mirocha JM, Bose N (2006) The Akt pathway in human breast cancer: a tissue-array-based analysis. Mod Pathol 19:238–245
Chuang HY, Lee E, Liu YT, Lee D, Ideker T (2007) Network-based classification of breast cancer metastasis. Mol Syst Biol 3:140
Deng J, Han Y, Yan C, Tian X, Tao J, Kang J, Li S (2010) Overexpressing cellular repressor of E1A-stimulated genes protects mesenchymal stem cells against hypoxia- and serum deprivation-induced apoptosis by activation of PI3K/Akt. Apoptosis 15:463–473
Desai BN, Myers BR, Schreiber SL (2002) FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA 99:4319–4324
Dillon RL, White DE, Muller WJ (2007) The phosphatidyl inositol 3-kinase signaling network: implications for human breast cancer. Oncogene 26:1338–1345
Dudkin L, Dilling MB, Cheshire PJ, Harwood FC, Hollingshead M, Arbuck SG, Travis R, Sausville EA, Houghton PJ (2001) Biochemical correlates of mTOR inhibition by the rapamycin ester CCI-779 and tumor growth inhibition. Clin Cancer Res 7:1758–1764
Finak G, Bertos N, Pepin F, Sadekova S, Souleimanova M, Zhao H, Chen H, Omeroglu G, Meterissian S, Omeroglu A, Hallett M, Park M (2008) Stromal gene expression predicts clinical outcome in breast cancer. Nat Med 14:518–527
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12:9–22
Heinonen H, Nieminen A, Saarela M, Kallioniemi A, Klefström J, Hautaniemi S et al (2008) Deciphering downstream gene targets of PI3K/mTOR/p70S6K pathway in breast cancer. BMC Genomics 9:348
Hernandez-Aya LF, Gonzalez-Angulo AM (2011) Targeting the phosphatidylinositol 3-kinase signaling pathway in breast cancer. Oncologist 16:(4)404–414
Macaskill EJ, Bartlett JM, Sabine VS, Faratian D, Renshaw L, White S, Campbell FM, Young O, Williams L, Thomas JS, Barber MD, Dixon JM (2011) The mammalian target of rapamycin inhibitor everolimus (RAD001) in early breast cancer: results of a pre-operative study. Breast Cancer Res Treat 128:725–734
Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM et al (2004) mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med 10:594–601
Meric-Bernstam F, Gonzalez-Angulo AM (2009) Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 27:2278–2287
Milani C, Welsh J, Katayama ML, Lyra EC, Maciel MS, Brentani MM, Folgueira MA (2010) Human breast tumor slices: a model for identification of vitamin D regulated genes in the tumor microenvironment. J Steroid Biochem Mol Biol 21:151–155
Mira-y-Lopez R, Osborne MP, DePalo AJ, Ossowski L (1991) Estradiol modulation of plasminogen activator production in organ cultures of human breast carcinomas: correlation with clinical outcome of anti-estrogen therapy. Int J Cancer 47:827–832
Noh WC, Mondesire WH, Peng J, Jian W, Zhang H, Dong J et al (2004) Determinants of rapamycin sensitivity in breast cancer cells. Clin Cancer Res 10:1013–1023
O’Reilly T, McSheehy PM (2010) Biomarker development for the clinical activity of the mTOR inhibitor everolimus (RAD001): processes, limitations, and further proposals. Transl Oncol 3:65–79
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D et al (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res 66:1500–1508
Park SS, Kim SW (2007) Activated Akt signaling pathway in invasive ductal carcinoma of the breast: correlation with HER2 overexpression. Oncol Rep 18:139–143
Pattingre S, Espert L, Biard-Piechaczyk M, Codogno P (2008) Regulation of macroautophagy by mTOR and beclin 1 complexes. Biochimie 90:313–323
Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC, Wolff NC, Tran TA, Zou L, Xie XJ, Corey DR, Brugarolas J (2011) Regulation of TFEB and V-ATPases by mTORC1. EMBO J 30:3242–3258
Peng T, Golub TR, Sabatini DM (2002) The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol 22:5575–5584
Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG, Stransky N, Tsun ZY, Cowley GS, Barretina J, Kalaany NY, Hsu PP, Ottina K, Chan AM, Yuan B, Garraway LA, Root DE, Mino-Kenudson M, Brachtel EF, Driggers EM, Sabatini DM (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476:346–350
Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J et al (2004) Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol 22:2336–2347
Rojo F, Najera L, Lirola J, Jiménez J, Guzmán M, Sabadell MD et al (2007) 4E-Binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res 13:81–89
Rozenchan PB, Carraro DM, Brentani H, de Carvalho Mota LD, Bastos EP, e Ferreira EN, Torres CH, Katayama ML, Roela RA, Lyra EC, Soares FA, Folgueira MA, Góes JC, Brentani MM (2009) Reciprocal changes in gene expression profiles of cocultured breast epithelial cells and primary fibroblasts. Int J Cancer 125:2767–2777
Sabine VS, Sims AH, Macaskill EJ, Renshaw L, Thomas JS, Dixon JM et al (2010) Gene expression profiling of response to mTOR inhibitor everolimus in pre-operatively treated post-menopausal women with oestrogen receptor-positive breast cancer. Breast Cancer Res Treat 122:419–428
Satheesha S, Cookson VJ, Coleman LJ, Ingram N, Madhok B, Hanby AM, Suleman CA, Sabine VS, Macaskill EJ, Bartlett JM, Dixon JM, McElwaine JN, Hughes TA (2011) Response to mTOR inhibition: activity of eIF4E predicts sensitivity in cell lines and acquired changes in eIF4E regulation in breast cancer. Mol Cancer 10:19
Settembre C, Zoncu R, Medina DL, Vetrini F, Erdin S, Erdin S, Huynh T, Ferron M, Karsenty G, Vellard MC, Facchinetti V, Sabatini DM, Ballabio A (2012) A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31:1095–1108
Sobral RA, Honda ST, Katayama ML, Brentani H, Brentani MM, Patrão DF, Folgueira MA (2008) Tumor slices as a model to evaluate doxorubicin in vitro treatment and expression of trios of genes PRSS11, MTSS1, CLPTM1 and PRSS11, MTSS1, SMYD2in canine mammary gland cancer. Acta Vet Scand 50:27
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Lønning PE, Børresen-Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA 98:10869–10874
Stoff-Khalili MA, Stoff A, Rivera AA, Banerjee NS, Everts M, Young S et al (2005) Preclinical evaluation of transcriptional targeting strategies for carcinoma of the breast in a tissue slice model system. Breast Cancer Res 7:1141–1152
Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon y Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J (2008) Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 26:1603–1610
Taylor IW, Linding R, Warde-Farley D, Liu Y, Pesquita C, Faria D, Bull S, Pawson T, Morris Q, Wrana JL (2009) Dynamic modularity in protein interaction networks predicts breast cancer outcome. Nat Biotechnol 27:199–204
Tokunaga E, Kimura Y, Oki E, Ueda N, Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Baba H, Maehara Y (2006) Akt is frequently activated in HER2/neu-positive breast cancers and associated with poor prognosis among hormone-treated patients.Int J Cancer 118:284–289
Trapé AP, Katayama ML, Roela RA, Brentani H, Ravacci GR, de Araujo LL, Brentani MM (2012) Gene expression profile in response to doxorubicin-rapamycin combined treatment of HER-2-overexpressing human mammary epithelial cell lines. Mol Cancer Ther 11:464–474
Vaira V, Fedele G, Pyne S, Fasoli E, Zadra G, Bailey D, Snyder E, Faversani A, Coggi G, Flavin R, Bosari S, Loda M (2010) Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci USA 107:8352–8356
Vestey SB, Sen C, Calder CJ, Perks CM, Pignatelli M, Winters ZE (2005) Activated Akt expression in breast cancer: correlation with p53, Hdm2 and patient outcome. Eur J Cancer 41:1017–1025
Yoo YA, Kang MH, Kim JS, Oh SC (2008) Sonic hedgehog signaling promotes motility and invasiveness of gastric cancer cells through TGF-beta-mediated activation of the ALK5-smad 3 pathway. Carcinogenesis 29:480–490
Yu K, Toral-Barza L, Discafani C, Zhang WG, Skotnicki J, Frost P, Gibbons JJ (2001) mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer 8:(3)249–258
Zhou X, Tan M, Stone Hawthorne V, Klos KS, Lan KH, Yang Y et al (2004) Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res 10:6779–6788
Zoncu R, Efeyan A, Sabatini DM (2011) mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 12:21–35
Acknowledgments
We are indebted to Dr. C. Krumdieck (University of Alabama, Birmingham, Ala., USA) for the kind donation of the Krumdieck tissue slicer to our institution. We also thank Dr. Igor Moyses Longo Snitcovsky for critical suggestions, Dr. Fiorita G. L. Mundim for helping with the immunohistochemical determinations, Mrs. Maria Jose Gonçalves Benevides for secretarial help and Mrs. Cristina Piñeiro Grandal for figure edition.
S.H.G.G. provided the tumor samples, prepared the tissue slices, collected all clinical data and was involved in drafting the manuscript. M.L.H.K. performed the culture, microarray and RT-PCR experiments. R.A.R. performed the microarray determinations. H.B. and R.A.R. performed the microarray data analysis. S.N., F.A.S. and A.F.L.W. participated in the immunohistochemistry studies. L.L. carried out the analysis of the co-expression network. J.C.S.G. provided clinical support for patient recruitment. M.A.A.K.F., M.L.H.K. and F.S.P. carried out the statistical analysis. M.M.B. was responsible for the study conception. M.M.B. and M.A.A.K.F. were involved in study design and manuscript preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
S.H.G.G., M.L.H.K. and R.A.R. contributed equally to this work.
This work was supported by FAPESP 2009/10088-7 and CNPQ.
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOC 316 kb)
Rights and permissions
About this article
Cite this article
Grosso, S.H.G., Katayama, M.L.H., Roela, R.A. et al. Breast cancer tissue slices as a model for evaluation of response to rapamycin. Cell Tissue Res 352, 671–684 (2013). https://doi.org/10.1007/s00441-013-1608-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1608-8