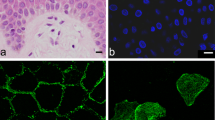
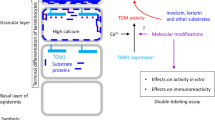
Abstract
Desquamation or cell shedding in mammalian skin is known to involve serine proteases, aspartic proteases and glycosidases. In addition, evidence continues to accumulate that papain-like cysteine proteases and an inhibitor cystatin M/E largely confined to the cutaneous epithelia also play key roles in the process. This involves the complete proteolysis of cell adhesive structures of the stratum corneum, the corneodesmosomes and notably of the desmogleins. Continual cell replacement in the epidermis is the result of the balance between the loss of the outer squames and mitosis of the cells in the basal cell layer. This article provides a brief account of the salient features of the characteristics and catalytic mechanism of cysteine proteases, followed by a discussion of the relevant epidermal biology. The proteases include the asparaginyl endopeptidase legumain, which exerts a strict specificity for the hydrolysis of asparaginyl bonds, cathepsin-V and cathepsin-L. The control of these enzymes by cystatin M/E regulates the processing of transglutaminases and is crucial in the biochemical pathway responsible for regulating the cross-linking and desquamation of the stratum corneum. In addition, caspase-14 has now been shown to play a major part in epidermal maturation. Uncontrolled proteolytic activity leads to abnormal hair follicle formation and deleterious effects on the skin barrier function.
Similar content being viewed by others
References
Abrahamson M, Alvarez-Fernandez M, Nathanson CM (2003) Cystatins. Biochem Soc Symp 70:179–199
Akgül B, Cooke JC, Storey A (2006) HPV-associated skin disease. J Pathol 208:165–175
Barrett AJ, Rawlings ND, Woessner JF (2004) Handbook of proteolytic enzymes. Elsevier, London
Benavides F, Starost MF, Flores M, Gimenez-Conti IB, Guenet JL, Conti CJ (2002) Impaired hair follicle morphogenesis and cycling with abnormal epidermal differentiation in nackt mice, a cathepsin L-deficient mutation. Am J Pathol 161:693–703
Blaydon DC, Nitoiu D, Eckl KM, Cabral RM, Bland P, Hausser I, Heel DA van, Rajpopat S, Fischer J, Oji V, Zvulunov A, Traupe H, Hennies HC, Kelsell DP (2011) Mutations in CSTA, encoding Cystatin A, underlie exfoliative ichthyosis and reveal a role for this protease inhibitor in cell-cell adhesion. Am J Hum Genet 89:564–571
Brocklehurst K (1982) Two–protonic-state electrophiles as probes of enzyme mechanism. Methods Enzymol 87:427–469
Brocklehurst K (1994) A sound basis for pH-dependent kinetic studies on enzymes. Protein Eng 7:291–299
Brocklehurst K, Little G (1970) A novel reactivity of papain and a convenient active site titration in the presence of other thiols. FEBS Lett 9:113–116
Brocklehurst K, Carlsson J, Kierston MPJ, Crook EM (1973) Covalent chromatography: preparation of fully active papain from dried papaya latex. Biochem J 133:573–584
Brocklehurst K, Carlsson J, Kierston MPJ, Crook EM (1974) Covalent chromatography by thiol-disulphide interchange. Methods Enzymol 34:531–544
Brocklehurst K, Carey PR, Lee H, Salih E, Storer AC (1984) Comparative resonance Raman spectroscopic and kinetic studies of acyl enzymes involving papain, actinidin and papaya peptidase II. Biochem J 233:649–657
Brocklehurst K, Willenbrook F, Salih E (1987) Cysteine proteinases. In: Neuberger A, Brocklehurst K (eds) Hydrolytic enzymes. New comprehensive biochemistry, vol 16. Elsevier, Amsterdam, pp 39–158
Brocklehurst K, Watts AB, Patel M, Verma C, Thomas EW (1998) Cysteine proteinases. In: Sinnott ML (ed) Comprehensive biological catalysis, vol 1. Academic Press, London, pp 381–423
Brocklehurst K, Courey AJ, Gul S, Lin S-H, Moritz RL (2004) Affinity and immunoaffinity chromatography. In: Simpson RJ (ed) Purifying proteins for proteomics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, pp 221–273
Brocklehurst K, Gul S, Pickersgill RW (2009) Substrate recognition. In: Hughes AB (ed) Amino acids peptides and proteins in organic chemistry, vol 2. Modified amino acids organocatalysis and enzymes. Wiley—VCH, Weinheim, pp 473–504
Candi E, Schmidt R, Melina G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6:328–340
Cheng T, Hitomi K, Ivonne MJ, Van Wlijmen-Willems J, Le Jongh GJ, Yamamoto K, Nishi K, Watts C, Reinheckel T, Schalkwijk J, Zeeuwen PL (2006) Cystatin M/E is a high affirmity inhibitor of cathepsin V and cathepsin L by a reactive site that is distinct from the legumain—binding site. A novel clue for the role of cystatin M/E in epidermal cornification. J Biol Chem 281:15893–15899
Cheng T, Tjabringa GS, Van Vlijmen-Willems IM, Hitomi K, Van Erp PE, Schalkwijk J, Zeeuwen PL (2009) The cystatin M/E controlled pathway of skin barrier formation: expression of its key components in psoriasis and atopic dermatitis. Br J Dermatol 161:253–264
Cornish-Bowden A (2002) Enthalpy-entropy compensation: a phantom phenomenon. J Biosci 27:121–126
Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S (2007) Caspase-14 protects against epidermal UVB photo damage and water loss. Nat Cell Biol 9:666–674
Denecker G, Ovaere P, Petal V (2008) Caspase-14 reveals its secrets. J Cell Biol 180:451–458
Dennemärker J, Lohmüller T, Mayerle J, Tacke M, Lerch MM, Coussens LM, Peters C, Reinheckel T (2010) Deficiency for the cysteine protease cathepsin L promotes tumor progression in mouse epidermis. Oncogene 29:1611–1621
Devos M, Prawitt J, Staumont-Salle D, Hoste E, Fleury S, Bouchaert E, Gilbert B, Lippens S, Vandenabeele P, Dombrowicz D, Declercq W (2012) Filaggrin degradation by caspase-14 is required for UVB photoprotection but does not influence allergic sensitization in a mouse model of atopic dermatitis. J Invest Dermatol 132:2857-2860
Drenth J, Kalk KH, Swen HM (1976) Binding of chloromethyl ketone substrate analogues to crystalline papain. Biochemistry 15:3731–3738
Dunitz JD (1995) Win some lose some: enthalpy-entropy compensation in weak intermolecular interactions. Chem Biol 2:709–712
Eckhart L, Declercq W, Ban J, Rendl M, Lengauer B, Mayer C, Lippens S, Vandenabeele P, Tschachler E (2000) Terminal differentiation of human keratinocytes and stratum corneum formation is associated with caspase-14 activation. J Invest Dermatol 115:1148–1151
Eckhart L, Ballaun C, Uthman A, Kittel C, Stichenwirth M, Buchberger M, Fischer H, Sipos W, Tschachler E (2005) Identification and characterization of a novel mammalian caspase with proapoptotic activity. J Biol Chem 280:35077–35080
Fischer H, Stichenwirth M, Dockal M, Ghannadan M, Buchberger M, Bach J, Kapetanopoulos A, Declercq W, Tschachler E, Eckhart L (2004) Stratum corneum-derived caspase-14 is catalytically active. FEBS Lett 577:446–450
Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, Dawson TM, Dawson VL, El-Deiry WS, Fulda S, Gottlieb E, Green DR, Hengartner MO, Kepp O, Knight RA, Kumar S, Lipton SA, Lu X, Madeo F, Malorni W, Mehlen P, Nuñez G, Peter ME, Piacentini M, Rubinsztein DC, Shi Y, Simon HU, Vandenabeele P, White E, Yuan J, Zhivotovsky B, Melino G, Kroemer G (2012) Molecular definitions of cell death subroutines: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 19:107–120
Gul G, Mellor GW, Thomas EW, Brocklehurst K (2006) Temperature dependences of the kinetics of the reactions of papain and actinidin with a series of recovering probes differing in key molecular recognition features. Biochem J 396:17–21
Gul G, Hussain S, Thomas MP, Resmini M, Verma CS, Thomas EW, Brocklehurst K (2008) Generation of nucleophilic character in the Cys 25/His 159 ion-pair of papain involves Trp177 but not Asp158. Biochemistry 47:2025–2035
Hagemann S, Gunther T, Dennemarker J, Lohmuller T, Bromme D, Schule R (2004) The human cysteine protease cathepsin V can compensate for murine cathepsin L in mouse epidermis and hair follicles. Eur J Cell Biol 83:775–780
Hene IB, Mattis JA, Fruton JS (1979) Interaction of papain with derivatives of phenylalanylglycinal: fluorescence studies. Proc Natl Acad Sci USA 76:1131–1134
Hussain S, Khan A, Brocklehurst K (2002) A review of developments in the study of cysteine proteinase mechanism: opportunities for investigation of electrostatic effects and dynamic aspects of molecular recognition in enzyme chemistry. Recent Res Dev Biochem 3:653–677
Hussain S, Pinitglang S, Bailey TSF, Reid JD, Noble MA, Resmini M, Thomas EW, Greaves RB, Verma CS, Brocklehurst K (2003) Variation in the pH-dependent pre-steady-state and steady-state kinetic characteristics of cysteine-proteinase mechanism: evidence for electrostatic modulation of catalytic-site function by the neighbouring carboxylate anion. Biochem J 372:735–746
Hussain S, Khan A, Gul S, Resmini M, Verma CS, Thomas EW, Brocklehurst K (2011) Identification of interactions involved in the generation of nucleophilic reactivity and of catalytic competence in the catalytic site Cys/His ion-pair of papain. Biochemistry 50:10732–10742
Huber CP, Ozaki Y, Pliura DH, Storer AC, Carey PR (1982) Precise structural information for transient enzyme-substrate complexes by a combined X-ray crystallographic-resonance Raman spectroscopic approach. Biochemisrry 21:3109-3115
Kato T, Takai T, Mitsuishi K, Okumura K, Ogawa H (2005) Cystatin A inhibits IL-8 production by keratinocytes stimulated with Der p 1 and Der f 1: biochemical skin barrier against mite cysteine proteases. J Allergy Clin Immunol 116:169–176
Kazem S, Meijden E van der, Struijk L, Gruijl FR de, Feltkamp MC (2012) Human papillomavirus 8 E6 disrupts terminal skin differentiation and prevents pro-Caspase-14 cleavage. Virus Res 163:609–616
Koenig U, Eckhart L, Tschachler E (2001) Evidence that caspase-13 is not a human but a bovine gene. Biochem Biophys Res Commun 285:1150–1154
Kovalenko A, Kim JC, Kang TB, Rajput A, Bogdanov K, Dittrich-Breiholz O, Kracht M, Brenner O, Wallach D (2009) Caspase-8 deficiency in epidermal keratinocytes triggers an inflammatory skin disease. J Exp Med 206:2161–2177
Kowlessur D, Topham CM, Thomas EW, O’Driscoll M, Templeton W, Brocklehurst K (1989) Identification of signalling and non-signalling binding contributions to enzyme reactivity. Alternate contributions of binding interactions provide for change in transition state geometry in reaction of papain. Biochem J 258:755–764
Krug RR, Hunter WG, Greiger RA (1976a) Enthalpy-entropy compensation. 1. Some fundamental statistical problems associated with the analysis of Van’t Hoff and Arrhenius data. J Phys Chem 80:2335–2340
Krug RR, Hunter WG, Greiger RA (1976b) Enthalpy-entropy compensation. 2. Separation of the chemical from the statistical effect. J Phys Chem 80:2341–2351
Lee P, Lee DJ, Chan C, Chen SW, Chen I, Jamora C (2009) Dynamic expression of epidermal caspase 8 simulates a wound healing response. Nature 458:519–523
Lippens S, Kockx M, Knaapen M, Mortier L, Polakowska R, Verheyen A, Garmyn M, Zwijsen A, Formstecher P, Huylebroeck D, Vandenabeele P, Declercq W (2000) Epidermal differentiation does not involve the pro-apoptotic executioner caspases, but is associated with caspase-14 induction and processing. Cell Death Differ 7:1218–1224
Lippens S, Kockx M, Denecker G, Knaapen M, Verheyen A, Christiaen R, Tschachler E, Vandenabeele P, Declercq W (2004) Vitamin D3 induces caspase-14 expression in psoriatic lesions and enhances caspase-14 processing in organotypic skin cultures. Am J Pathol 165:833–841
Lowe G (1976) The cysteine proteinases. Tetrahedron 32:291–302
Lowe G, Williams A (1965) Direct evidence for an acylated thiol as an intermediate in papain– and ficin–catalysed hydrolysis. Biochem J 96:189–193
Mellor GW, Thomas EW, Topham CM, Brocklehurst K (1993) The ionization characteristics of the Cys 25–His 159 interactive system and of the modulatory group of papain. Resolution of ambiguity by electronic perturbation of the quasi-2-mercaptopyridine leaving group in a new pyrimidyl disulphide reactivity probe. Biochem J 290:75–83
Menard R, Carriere J, Laflamme P, Plouffe C, Khouri H, Vernet T, Tessier DC, Thomas DY, Storer AC (1991) Contribution of the glutamine 19 sidechain to transition state stabilization in the oxyarion hole of papain. Biochemistry 30:8924–8928
Menard R, Plouffe C, Laflamme P, Vernet T, Tessier DC, Thomas DY, Storer AC (1995) Modification of the electrostatic environment is tolerated in the oxyarion hole of the cysteine protease papain. Biochemistry 34:464–471
Mielgo A, Torres VA, Schmid MC, Graf R, Zeitlin SG, Lee P, Shields DJ, Barbero S, Jamora C, Stupack DG (2009) The death effector domains of caspase-8 induce terminal differentiation. PLoS One 4:e7879
Noble MA, Gul S, Verma CS, Brocklehurst K (2000) The ionization characteristics and chemical influences of aspartic acid residue 158 of papain and caricain determined by structure-related kinetic and computational techniques: multiple electrostatic modulators of active centre chemistry. Biochem J 351:723–733
Patel M, Kayani S, Templeton W, Mellor GW, Thomas EW, Brocklehurst K (1992) Evaluation of hydrogen bonding and enantiomeric P2-S2 hydrophobic contacts in dynamic aspects of molecular recognition by papain. Biochem J 287:881–889
Pinitglang S, Watts AB, Patel M, Reid JD, Noble MA, Gul S, Bokth A, Naeem A, Patel H, Thomas EW, Sreedharan S, Verma C, Brocklehurst K (1997) A classical enzyme active centre motif lacks catalytic competence until modulated electrostatically. Biochemistry 36:9968–9982
Raymond AA, Méchin MC, Nachat R, Toulza E, Tazi-Ahnini R, Serre G, Simon M (2007) Nine procaspases are expressed in normal human epidermis, but only caspase-14 is fully processed. Br J Dermatol 156:420–427
Reid JD, Hussain S, Sreedharan SK, Bailey TSF, Pinitglan S, Thomas EW, Verma CS, Brocklehurst K (2001) Variation in aspects of cysteine proteinase catalytic mechanism deduced spectroscopic observation of dithioester intermediates, kinetic analysis and molecular dynamics simulations. Biochem J 357:343–352
Reinheckel T, Hagemann S, Dollwet-Mack S, Martinez E, Lohmuller T, Zlatkovic G (2005) The lysosomal cysteine protease cathepsin L regulates keratinocyte proliferation by control of growth factor recycling. J Cell Sci 118:3387–3395
Roth W, Deussing J, Botchkarev VA, Pauly-Evers M, Saftig P, Hafner A (2000) Cathepsin L deficiency as molecular defect of furless: hyperproliferation of keratinocytes and pertubation of hair follicle cycling. FASEB J 14:2075–2086
Rubach JK, Cui G, Schneck JL, Taylor AN, Zhao B, Smallwood A, Nevins N, Wisnoski D, Thrall SH, Meek TD (2012) The amino-acid substituents of dipeptide substrates of cathepsin C can determine the rate-limiting steps of catalysis.Biochemistry (in press)
Samuelsson L, Stiller C, Friberg C, Nilsson C, Inerot A, Wahlström J (2004) Association analysis of cystatin A and zinc finger protein 148, two genes located at the psoriasis susceptibility locus PSORS5. J Invest Dermatol 122:1399–1400
Simpson RJ (2004) Purifying proteins for proteomics: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Smith FJ, Irvine AD, Terron-Kwiatkowski A, Sandilands A, Campbell LE, Zhao Y (2006) Loss-of-function mutations in the gene encoding filaggrin cause ichthyosis vulgaris. Nat Genet 38:337–342
Steven AC, Steinert PM (1994) Protein composition of cornified cell envelopes of epidermal keratinocytes. J Cell Sci 107:693–700
Storer AC, Lee H, Carey PR (1983) Relaxed and perturbed substrate conformations in enzyme active sites: evidence from multichannel resonance Raman spectra.Biochemistry 22:4789-4796
Sundberg JP, Boggess D, Hogan ME, Sundberg BA, Rourk MH, Harris B (1997) Harlequin ichthyosis (ichq): a juvenile lethal mouse mutation with ichthyosiform dermatitis. Am J Pathol 151:293–310
Tholen S, Biniossek ML, Gansz M, Gomez-Auli A, Werner F, Noel A, Kizhakkedathu JN, Boerries M, Busch H, Reinheckel T, Schilling O (2012) Deletion of cysteine cathepsins B or L yields differential impacts on murine skin proteome and degradome. Mol Cell Proteomics (in press)
Turk V, Turk B, Turk D (2001) Lysosomal cysteine proteases: facts and opportunities.EMBO J 20:4629-4633
Van de Craen M, Vandenabeele P, Declercq W, Van den Brande I, Van Loo G, Molemans F, Schotte P, Van Criekinge W, Beyaert R, Fiers W (1997) Characterization of seven murine caspase family members. FEBS Lett 403:61–69
Vasilopoulos Y, Sagoo GS, Cork MJ, Walters K, Tazi-Ahnini R (2011) HLA-C, CSTA and DS12346 susceptibility alleles confer over 100-fold increased risk of developing psoriasis: evidence of gene interaction. J Hum Genet 56:423–427
Wu NL, Lee TA, Tsai TL, Lin WW (2011) TRAIL-induced keratinocyte differentiation requires caspase activation and p63 expression. J Invest Dermatol 131:874–883
Yuan J (2006) Divergence from a dedicated cellular suicide mechanism: exploring the evolution of cell death. Mol Cell 23:1–12
Zeeuwen PL, Vlijmen-Willems IM, Jansen BJ, Sotiropoulou G, Curfs JH, Meis JF (2001) Cystatin M/E expression is restricted to differentiated epidermal keratinocytes and sweat glands: a new skin-specific proteinase inhibitor that is a target for cross-linking by transglutaminase. J Invest Dermatol 116:693–701
Zeeuwen PL, Vlijmen-Willems IM, Hendriks W (2002) A null mutation in the cystatin M/E gene of ichq mice causes juvenile lethality and defects in epidermal cornification. Hum Mol Genet 11:2867–2875
Zeeuwen PL, Dale BA, De Jongh G, Vlijmen-Willems IM, Fleckman P, Kimball JR (2003) The human cystatin M/E gene (CST6): exclusion as a candidate gene for harlequin ichthyosis. Invest Dermatol 121:65–68
Zeeuwen PL, Van Vlijmen-Willems IM, Olthuis D, Johansen HT, Hitomi K, Hara-Nishimura I, Powers JC, James KE, Camp HJ op den, Lemmens R, Schalkwijk J (2004) Evidence that unrestricted legumain activity is involved in disturbed epidermal cornification in cystatin M/E deficient mice.Hum Mol Genet 13:1069-1079
Zeeuwen PLJM, Ishida-Yamamoto A, Van Vlijmen-Willems IMJJ, Cheng T, Berges M, Iizuka H, Schalwijk J (2007) Colocalization of cystatin M/E and cathepsin V in lamellar granules and corneodesmosomes suggests a functional role in epidermal differentiation. J Invest Dermatol 127:120–128
Zeeuwen PL, Cheng T, Schalwijk J (2009) The biology of cystatin M/E and its cognate target protease. J Invest Dermatol 129:1327–1338
Zeeuwen PL, Van Vlijmen-Willems IM, Cheng T, Rodijk-Olthuis D, Hitomi K, Hara-Nishimura I, John S, Smyth N, Reinheckel T, Hendriks WJ, Schalkwijk J (2010) The cystatin M/E-cathepsin L balance is essential for tissue homeostasis in epidermis, hair follicles, and cornea. FASEB J 24:3744–3755
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brocklehurst, K., Philpott, M.P. Cysteine proteases: mode of action and role in epidermal differentiation. Cell Tissue Res 351, 237–244 (2013). https://doi.org/10.1007/s00441-013-1557-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-013-1557-2