Abstract
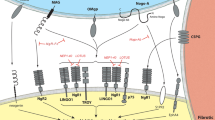
Limited axonal plasticity within the central nervous system (CNS) is a major restriction for functional recovery after CNS injury. The small GTPase RhoA is a key molecule of the converging downstream cascade that leads to the inhibition of axonal re-growth. The Rho-pathway integrates growth inhibitory signals derived from extracellular cues, such as chondroitin sulfate proteoglycans, Nogo-A, myelin-associated glycoprotein, oligodendrocyte-myelin glycoprotein, Ephrins and repulsive guidance molecule-A, into the damaged axon. Consequently, the activation of RhoA results in growth cone collapse and finally outgrowth failure. In turn, the inhibition of RhoA-activation blinds the injured axon to its growth inhibitory environment resulting in enhanced axonal sprouting and plasticity. This has been demonstrated in various CNS-injury models for direct RhoA-inhibition and for downstream/upstream blockade of the RhoA-associated pathway. In addition, RhoA-inhibition reduces apoptotic cell death and secondary damage and improves locomotor recovery in clinically relevant models after experimental spinal cord injury (SCI). Unexpectedly, a subset of “small molecules” from the group of non-steroid anti-inflammatory drugs, particularly the FDA-approved ibuprofen, has recently been identified as (1) inhibiting RhoA-activation, (2) enhancing axonal sprouting/regeneration, (3) protecting “tissue at risk” (neuroprotection) and (4) improving motor recovery confined to realistic therapeutical time-frames in clinically relevant SCI models. Here, we survey the effect of small-molecule-induced RhoA-inhibition on axonal plasticity and neurofunctional outcome in CNS injury paradigms. Furthermore, we discuss the body of preclinical evidence for a possible clinical translation with a focus on ibuprofen and illustrate putative risks and benefits for the treatment of acute SCI.


Similar content being viewed by others
References
Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M (2008) PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science 322:967–970
Barton WA, Liu BP, Tzvetkova D, Jeffrey PD, Fournier AE, Sah D, Cate R, Strittmatter SM, Nikolov DB (2003) Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins. EMBO J 22:3291–3302
Bartus K, James ND, Bosch KD, Bradbury EJ (2011) Chondroitin sulphate proteoglycans: key modulators of spinal cord and brain plasticity. Exp Neurol. doi:10.1016/j.expneurol.2011.08.008
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21
Basso DM, Fisher LC, Anderson AJ, Jakeman LB, McTigue DM, Popovich PG (2006) Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23:635–659
Benson MD, Romero MI, Lush ME, Lu QR, Henkemeyer M, Parada LF (2005) Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc Natl Acad Sci USA 102:10694–10699
Boato F, Hendrix S, Huelsenbeck SC, Hofmann F, Grosse G, Djalali S, Klimaschewski L, Auer M, Just I, Ahnert-Hilger G, Höltje M (2010) C3 peptide enhances recovery from spinal cord injury by improved regenerative growth of descending fiber tracts. J Cell Sci 123:1652–1662
Borisoff JF, Chan CC, Hiebert GW, Oschipok L, Robertson GS, Zamboni R, Steeves JD, Tetzlaff W (2003) Suppression of Rho-kinase activity promotes axonal growth on inhibitory CNS substrates. Mol Cell Neurosci 22:405–416
Buchli AD, Schwab ME (2005) Inhibition of Nogo: a key strategy to increase regeneration, plasticity and functional recovery of the lesioned central nervous system. Ann Med 37:556–567
Center for Drug Evaluation and Research, FDA (2005) Guidance for industry, estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult health volunteers. www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm078932.pdf
Chacon PJ, Garcia-Mejias R, Rodriguez-Tebar A (2011) Inhibition of RhoA GTPase and the subsequent activation of PTP1B protects cultured hippocampal neurons against amyloid β toxicity. Mol Neurodegener 6:14
Chan CC, Khodarahmi K, Liu J, Sutherland D, Oschipok LW, Steeves JD, Tetzlaff W (2005) Dose-dependent beneficial and detrimental effects of ROCK inhibitor Y27632 on axonal sprouting and functional recovery after rat spinal cord injury. Exp Neurol 196:352–364
Chan CC, Wong AK, Liu J, Steeves JD, Tetzlaff W (2007) ROCK inhibition with Y27632 activates astrocytes and increases their expression of neurite growth-inhibitory chondroitin sulfate proteoglycans. Glia 55:369–384
Conrad S, Genth H, Hofmann F, Just I, Skutella T (2007) Neogenin-RGMa signaling at the growth cone is bone morphogenetic protein-independent and involves RhoA, ROCK, and PKC. J Biol Chem 282:16423–16433
Curt A, Hedel HJ van, Klaus D, Dietz V, EM-SCI Study Group (2008) Recovery from a spinal cord injury: significance of compensation, neural plasticity, and repair. J Neurotrauma 25:677–685
Davies NM (1998) Clinical pharmacokinetics of iburofen. The first 30 years. Clin Pharmacokinet 34:101–154
Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L (2002) Rho signaling pathway targeted to promote spinal cord repair. J Neurosci 22:6570–6577
Detloff MR, Fisher LC, McGaughy V, Longbrake EE, Popovich PG, Basso DM (2008) Remote activation of microglia and pro-inflammatory cytokines predict the onset and severity of below-level neuropathic pain after spinal cord injury in rats. Exp Neurol 212:337–347
Dill J, Patel AR, Yang XL, Bachoo R, Powell CM, Li S (2010) A molecular mechanism for ibuprofen-mediated RhoA inhibition in neurons. J Neurosci 30:963–972
Ding J, Li QY, Wang X, Sun CH, Lu CZ, Xiao BG (2010) Fasudil protects hippocampal neurons against hypoxia-reoxygenation injury by suppressing microglial inflammatory responses in mice. J Neurochem 114:1619–1629
Dubreuil CI, Winton MJ, McKerracher L (2003) Rho activation patterns after spinal cord injury and the role of activated Rho in apoptosis in the central nervous system. J Cell Biol 162:233–243
Duffy P, Schmandke A, Sigworth J, Narumiya S, Cafferty WB, Strittmatter SM (2009) Rho-associated kinase II (ROCKII) limits axonal growth after trauma within the adult mouse spinal cord. J Neurosci 29:15266–15276
Fehlings MG, Theodore N, Harrop J, Maurais G, Kuntz C, Shaffrey CI, Kwon BK, Chapman J, Yee A, Tighe A, McKerracher L (2011) A phase I/IIa clinical trial of a recombinant Rho protein antagonist in acute spinal cord injury. J Neurotrauma 28:787–796
Fournier AE, GrandPre T, Strittmatter SM (2001) Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature 409:341–346
Fournier AE, Gould GC, Liu BP, Strittmatter SM (2002) Truncated soluble Nogo receptor binds Nogo-66 and blocks inhibition of axon growth by myelin. J Neurosci 22:8876–8883
Fournier AE, Takizawa BT, Strittmatter SM (2003) Rho kinase inhibition enhances axonal regeneration in the injured CNS. J Neurosci 23:1416–1423
Fu Q, Hue J, Li S (2007) Nonsteroidal anti-inflammatory drugs promote axon regeneration via RhoA inhibition. J Neurosci 27:4154–4164
Fujita Y, Endo S, Takai T, Yamashita T (2011) Myelin suppresses axon regeneration by PIR-B/SHP-mediated inhibition of Trk activity. EMBO J 30:1389–1401
Goldshmit Y, Spanevello MD, Tajouri S, Li L, Rogers F, Pearse M, Galea M, Bartlett PF, Boyd AW, Turnley AM (2011) EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice. PLoS One 6:e24636
GrandPré T, Li S, Strittmatter SM (2002) Nogo-66 receptor antagonist peptide promotes axonal regeneration. Nature 417:547–551
Guo LH, Trautmann K, Schluesener HJ (2005) Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol 163:120–127
Guth L, Zhang Z, DiProspero NA, Joubin K, Fitch MT (1994) Spinal cord injury in the rat: treatment with bacterial lipopolysaccharide and indomethacin enhances cellular repair and locomotor function. Exp Neurol 126:76–87
Hoffmann A, Hofmann F, Just I, Lehnardt S, Hanisch UK, Brück W, Kettenmann H, Ahnert-Hilger G, Höltje M (2008) Inhibition of Rho-dependent pathways by Clostridium botulinum C3 protein induces a proinflammatory profile in microglia. Glia 56:1162–1175
Hu F, Strittmatter SM (2008) The N-terminal domain of Nogo-A inhibits cell adhesion and axonal outgrowth by an integrin-specific mechanism. J Neurosci 28:1262–1269
Huang L, He Z, Guo L, Wang H (2008) Improvement of cognitive deficit and neuronal damage in rats with chronic cerebral ischemia via relative long-term inhibition of rho-kinase. Cell Mol Neurobiol 28:757–768
Huebner EA, Kim BG, Duffy PJ, Brown RH, Strittmatter SM (2011) A multi-domain fragment of Nogo-A protein is a potent inhibitor of cortical axon regeneration via Nogo receptor 1. J Biol Chem 286:18026–18036
Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H (2004) Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 10:712–718
Jin Z, Strittmatter SM (1997) Rac1 mediates collapsin-1-induced growth cone collapse. J Neurosci 17:6256–6263
Julien S, Schnichels S, Teng H, Tassew N, Henke-Fahle S, Mueller BK, Monnier PP (2008) Purkinje cell survival in organotypic cultures: implication of Rho and its downstream effector ROCK. J Neurosci Res 86:531–536
Konstan MW (2008) Ibuprofen therapy for cystic fibrosis lung disease: revisited. Curr Opin Pulm Med 14:567–573
Kottis V, Thibault P, Mikol D, Xiao Z-C, Zhang R, Dergham P, Braun PE (2002) Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J Neurochem 82:1566–1569
Kuijk AA van, Geurts AC, Kuppevelt HJ van (2008) Neurogenic heterotopic ossification in spinal cord injury. Spinal Cord 40:313–326
Kwon BK, Oxland TR, Tetzlaff W (2002) Animal models used in spinal cord regeneration research. Spine 27:1504–1510
Lands LC, Stanojevic S (2007) Oral non-steroidal anti-inflammatory drug therapy for cystic fibrosis. Cochrane Database Syst Rev 4:CD001505
Lee JK, Kim JE, Sivula M, Strittmatter SM (2004) Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci 24:6209–6217
Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, Tigyi G, McKerracher L (1999) Inactivation of Rho signaling pathway promotes CNS axon regeneration. J Neurosci 19:7537–7547
Li S, Strittmatter SM (2003) Delayed systemic Nogo-66 receptor antagonist promotes recovery from spinal cord injury. J Neurosci 23:4219–4227
Li S, Liu BP, Budel S, Li M, Ji B, Walus L, Li W, Jirik A, Rabacchi S, Choi E, Worley D, Sah DW, Pepinsky B, Lee D, Relton J, Strittmatter SM (2004) Blockade of Nogo-66, myelin-associated glycoprotein, and oligodendrocyte myelin glycoprotein by soluble Nogo-66 receptor promotes axonal sprouting and recovery after spinal injury. J Neurosci 24:10511–10520
Li Q, Huang XJ, He W, Ding J, Jia JT, Fu G, Wang HX, Guo LJ (2009) Neuroprotective potential of fasudil mesylate in brain ischemia-reperfusion injury of rats. Cell Mol Neurobiol 29:169–180
Liebscher T, Schnell L, Schnell D, Scholl J, Schneider R, Gullo M, Fouad K, Mir A, Rausch M, Kindler D, Hamers FP, Schwab ME (2005) Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol 58:706–719
Lim GP, Yang F, Chu T, Gahtan E, Ubeda O, Beech W, Overmier JB, Hsiao-Ashec K, Frautschy SA, Cole GM (2001) Ibuprofen effects on Alzheimer pathology and open field activity in APPsw transgenic mice. Neurobiol Aging 22:983–991
Liu BP, Fournier A, GrandPre T, Strittmatter SM (2002) Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science 297:1190–1193
Liu BP, Cafferty WB, Budel SO, Strittmatter SM (2006) Extracellular regulators of axonal growth in the adult central nervous system. Philos Trans R Soc Lond Biol 361:1593–1610
Lord-Fontaine S, Yang F, Diep Q, Dergham P, Munzer S, Tremblay P, McKerracher L (2008) Local inhibition of Rho signaling by cell-permeable recombinant protein BA-210 prevents secondary damage and promotes functional recovery following acute spinal cord injury. J Neurotrauma 25:1309–1322
Mandula H, Parepally JM, Feng R, Smith QR (2006) Role of site-specific binding to plasma albumin in drug availability to brain. J Pharmacol Exp Ther 317:667–675
McGee AW, Strittmatter SM (2003) The Nogo-66 receptor: focusing myelin inhibition of axon regeneration. Trends Neurosci 26:193–198
McKerracher L, Higuchi H (2006) Targeting Rho to stimulate repair after spinal cord injury. J Neurotrauma 23:309–317
McTigue DM (2008) Potential therapeutic targets for PPARgamma after spinal cord injury. PPAR Res 2008:517162
Merkler D, Metz GA, Raineteau O, Dietz V, Schwab ME, Fouad K (2001) Locomotor recovery in spinal cord-injured rats treated with an antibody neutralizing the myelin-associated neurite growth inhibitor Nogo-A. J Neurosci 21:3665–3673
Metz GA, Curt A, Meent H van de, Klusman I, Schwab ME, Dietz V (2000a) Validation of the weight-drop contusion model in rats: a comparative study of human spinal cord injury. J Neurotrauma 17:1–17
Metz GA, Merkler D, Dietz V, Schwab ME, Fouad K (2000b) Efficient testing of motor function in spinal cord injured rats. Brain Res 883:165–177
Mi S, Lee X, Shao ZH, Thill G, Ji BX, Relton J, Levesque M, Allaire N, Perrin S, Sands B, Crowell T, Cate RL, McCoy JM, Pepinsky RB (2004) LINGO-1 is a component of the Nogo-66 receptor/p75 signaling complex. Nat Neurosci 7:221–228
Milligan ED, Watkins LR (2009) Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci 10:23–36
Mills RF, Adams SS, Cliffe EE, Dickinson W, Nicholson JS (1973) The metabolism of ibuprofen. Xenobiotica 3:589–598
Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK (2003) The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 22:319–330
Morgenstern DA, Asher RA, Fawcett JW (2002) Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res 137:313–332
Mueller BK, Mack H, Teusch N (2005) Rho kinase, a promising drug target for neurological disorders. Nat Rev Drug Discov 4:387–398
Muellner A, Gonzenbach RR, Weinmann O, Schnell L, Liebscher T, Schwab ME (2008) Lamina-specific restoration of serotonergic projections after Nogo-A antibody treatment of spinal cord injury in rats. Eur J Neurosci 27:326–333
Nakamura Y, Fujita Y, Ueno M, Takai T, Yamashita T (2011) Paired immunoglobulin-like receptor B knockout does not enhance axonal regeneration or locomotor recovery after spinal cord injury. J Biol Chem 286:1876–1883
Ohsawa M, Aasato M, Hayashi SS, Kamei J (2011) RhoA/Rho kinase pathway contributes to the pathogenesis of thermal hyperalgesia in diabetic mice. Pain 152:114–122
Omoto S, Ueno M, Mochio S, Takai T, Yamashita T (2010) Genetic deletion of paired immunoglobulin-like receptor B does not promote axonal plasticity or functional recovery after traumatic brain injury. J Neurosci 30:13045–13052
Otsuka S, Adamson C, Sankar V, Gibbs KM, Kane-Goldsmith N, Ayer J, Babiarz J, Kalinski H, Ashush H, Alpert E, Lahav R, Feinstein E, Grumet M (2011) Delayed intrathecal delivery of RhoA siRNA to the contused spinal cord inhibits allodynia, preserves white matter, and increases serotonergic fiber growth. J Neurotrauma 28:1063–1076
Pantovic R, Draganic P, ErakovicV BB, Milin C, Simonic A (2005) Effect of indomethacin on motor activity and spinal cord free fatty acid content after experimental spinal cord injury in rabbits. Spinal Cord 43:519–526
Parepally JM, Mandula H, Smith QR (2006) Brain uptake of nonsteroidal anti-inflammatory drugs: ibuprofen, flurbiprofen, and indomethacin. Pharm Res 23:873–881
Rajagopalan S, Deitinghoff L, Davis D, Conrad S, Skutella T, Chedotal A, Mueller BK, Strittmatter SM (2004) Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol 6:756–762
Ramer LM, Borisoff JF, Ramer MS (2004) Rho-kinase inhibition enhances axonal plasticity and attenuates cold hyperalgesia after dorsal rhizotomy. J Neurosci 24:10796–10805
Rikitake Y, Kim HH, Huang Z, Seto M, Yano K, Asano T, Moskowitz MA, Liao JK (2005) Inhibition of Rho kinase (ROCK) leads to increased cerebral blood flow and stroke protection. Stroke 36:2251–2257
Robak LA, Venkatesh K, Lee H, Raiker SJ, Duan Y, Lee-Osbourne J, Hofer T, Mage RG, Rader C, Giger RJ (2009) Molecular basis of the interactions of the Nogo-66 receptor and its homolog NgR2 with myelin-associated glycoprotein: development of NgROMNI-Fc, a novel antagonist of CNS myelin inhibition. J Neurosci 29:5768–5783
Rossignol S, Schwab M, Schwartz M, Fehlings MG (2007) Spinal cord injury: time to move? J Neurosci 27:11782–11792
Satoh S, Ikegaki I, Suzuki Y, Asano T, Shibuya M, Hidaka H (1996) Neuroprotective properties of a protein kinase inhibitor against ischaemia-induced neuronal damage in rats and gerbils. Br J Pharmacol 118:1592–1596
Satoh S, Toshima Y, Hitomi A, Ikegaki I, Seto M, Asano T (2008) Wide therapeutic time window for Rho-kinase inhibition therapy in ischemic brain damage in a rat cerebral thrombosis model. Brain Res 1193:102–108
Schafer AI (1999) Effects of nonsteroidal anti-inflammatory therapy on platelets. Am J Med 106:25S–36S
Scheuren N, Bang H, Münster T, Brune K, Pahl A (1998) Modulation of transcription factor NF-kappaB by enantiomers of the nonsteroidal drug ibuprofen. Br J Pharmacol 123:645–652
Schmandke A, Schmandke A, Strittmatter SM (2007) ROCK and Rho: biochemistry and neuronal functions of Rho-associated protein kinases. Neuroscientist 13:454–469
Schwab JM, Hirsch S, Monnier PP, Brechtel K, Stiefel A, Leppert CA, Schluesener HJ, Barth H, Aktories K, Mueller BK (2002) The Rho-GTPase inhibitor C3-C2IN/C2II induces functional neuronal recovery in a rat model of severe spinal cord injury. Program No. 204.7. Abstract Viewer/Itinerary Planner. Society for Neuroscience, Washington, DC
Schwab JM, Conrad S, Elbert T, Trautmann K, Meyermann R, Schluesener HJ (2004) Lesional RhoA + cell numbers are suppressed by anti-inflammatory, cyclooxygenase-inhibiting treatment following subacute spinal cord injury. Glia 47:377–386
Schwab JM, Guo L, Schluesener HJ (2005) Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol 163:185–189
Schwab JM, Tuli SK, Failli V (2006) The Nogo receptor complex: confining molecules to molecular mechanisms. Trends Mol Med 12:293–297
Schweigreiter R, Bandtlow CE (2006) Nogo in the injured spinal cord. J Neurotrauma 23:384–396
Seymour AB, Andrews EM, Tsai SY, Markus TM, Bollnow MR, Brenneman MM, O'Brien TE, Castro AJ, Schwab ME, Kartje GL (2005) Delayed treatment with monoclonal antibody IN-1 1 week after stroke results in recovery of function and corticorubral plasticity in adult rats. J Cereb Blood Flow Metab 25:1366–1375
Shao Z, Browning JL, Lee X, Scott ML, Shulga-Morskaya S, Allaire N, Thill G, Levesque M, Sah D, McCoy JM, Murray B, Jung V, Pepinsky RB, Mi S (2005) TAJ/TROY, an orphan TNF receptor family member, binds Nogo-66 receptor 1 and regulates axonal regeneration. Neuron 45:353–359
Sharma HS, Winkler T (2002) Assessment of spinal cord pathology following trauma using early changes in the spinal cord evoked potentials: a pharmacological and morphological study in the rat. Muscle Nerve 25 (Suppl 11):S83–S91
Sharma HS, Olsson Y, Cervos-Navarro J (1993a) Early perifocal cell changes and edema in traumatic injury of the spinal cord are reduced by indomethacin, an inhibitor of prostaglandin synthesis. Experimental study in the rat. Acta Neuropathol 85:145–153
Sharma HS, Olsson Y, Nyberg F, Dey PK (1993b) Prostaglandins modulate alterations of microvascular permeability, blood flow, edema and serotonin levels following spinal cord injury: an experimental study in the rat. Neuroscience 57:443–449
Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG (2009) PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592–596
Shibuya M, Hirai S, Seto M, Satoh S, Ohtomo E, Fasudil Ischemic Stroke Study Group (2005) Effects of fasudil in acute ischemic stroke: results of a prospective placebo-controlled double-blind trial. J Neurol Sci 238:31–39
Shin HK, Salomone S, Potts EM, Lee SW, Millican E, Noma K, Huang PL, Boas DA, Liao JK, Moskowitz MA, Ayata C (2007) Rho-kinase inhibition acutely augments blood flow in focal cerebral ischemia via endothelial mechanisms. J Cereb Blood Flow Metab 27:998–1009
Siddall PJ, Loeser JD (2001) Pain following spinal cord injury. Spinal Cord 39:63–73
Siddall PJ, Taylor DA, McClelland JM, Rutkowski SB, Cousins MJ (1999) Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain 81:187–197
Simpson RK Jr, Baskin DS, Dudley AW, Bogue L, Rothenberg F (1991) The influence of long-term nifedipine or indomethacin therapy on neurologic recovery from experimental spinal cord injury. J Spinal Disord 4:420–427
Sing G, Ramey DR (1998) NSAID induced gastrointestinal complications: The ARAMIS Perspective-1997. J Rheumatol 25 (Suppl 51):8–16
Tanaka H, Yamashita T, Yachi K, Fujiwara T, Yoshikawa H, Tohyama M (2004) Cytoplasmic p21(Cip1/WAF1) enhances axonal regeneration and functional recovery after spinal cord injury in rats. Neuroscience 127:155–164
Tator CH (2006) Review of treatment trials in human spinal cord injury: issues, difficulties, and recommendations. Neurosurgery 59:957–982
Tatsumi S, Mabuchi T, Katano T, Matsumura S, Abe T, Hidaka H, Suzuki M, Sasaki Y, Minami T, Ito S (2005) Involvement of Rho-kinase in inflammatory and neuropathic pain through phosphorylation of myristoylated alanine-rich C-kinase substrate (MARCKS). Neuroscience 131:491–498
Thoenen H, Sendtner M (2002) Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat Neurosci 5 (Suppl):1046–1050
Toshima Y, Satoh S, Ikegaki I, Asano T (2000) A new model of cerebral microthrombosis in rats and the neuroprotective effect of a Rho-kinase inhibitor. Stroke 31:2245–2250
Tsuda M, Shigemoto-Mogami Y, Koizumi S, Mizokoshi A, Kohsaka S, Salter MW, Inoue K (2003) P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424:778–783
Tsuda M, Toyomitsu E, Komatsu T, Masuda T, Kunifusa E, Nasu-Tada K, Koizumi S, Yamamoto K, Ando J, Inoue K (2008) Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia 56:579–585
Wang KC, Kim JA, Sivasankaran R, Segal R, He Z (2002) P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature 420:74–78
Wang X, Baughman KW, Basso DM, Strittmatter SM (2006) Delayed Nogo receptor therapy improves recovery from spinal cord contusion. Ann Neurol 60:540–549
Wang X, Budel S, Baughman GG, Song KH, Strittmatter SM (2009) Ibuprofen enhances recovery from spinal cord injury by limiting tissue loss and stimulating axonal growth. J Neurotrauma 26:81–95
Weggen S, Eriksen JL, Das P, Sagi SA, Wang R, Pietrzik CU, Findlay KA, Smith TE, Murphy MP, Bulter T, Kang DE, Marquez-Sterling N, Golde TE, Koo EH (2001) A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature 414:212–216
Weggen S, Eriksen JL, Sagi SA, Pietrzik CU, Golde TE, Koo EH (2003) Abeta42-lowering nonsteroidal anti-inflammatory drugs preserve intramembrane cleavage of the amyloid precursor protein (APP) and ErbB-4 receptor and signaling through the APP intracellular domain. J Biol Chem 278:30748–30754
Whelton A (1999) Nephrotoxicity of nonstreoidal anti-inflammatory drugs: physiologic foundations and clinical implications. Am J Med 106:13S–24S
Winkler T, Sharma HS, Stalberg E, Olsson Y (1993) Indomethacin, an inhibitor of prostaglandin synthesis attenuates alteration in spinal cord evoked potentials and edema formation after trauma to the spinal cord: an experimental study in the rat. Neuroscience 52:1057–1067
Wrathall JR, Pettegrew RK, Harvey F (1985) Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol 88:108–122
Wu D, Yang P, Zhang X, Luo J, Haque ME, Yeh J, Richardson PM, Zhang Y, Bo X (2009) Targeting a dominant negative rho kinase to neurons promotes axonal outgrowth and partial functional recovery after rat rubrospinal tract lesion. Mol Ther 17:2020–2030
Xie F, Zheng B (2008) White matter inhibitors in CNS axon regeneration failure. Exp Neurol 209:302–312
Xing B, Li H, Wang H, Mukhopadhyay D, Fisher D, Gilpin CJ, Li S (2011) RhoA-inhibiting NSAIDs promote axonal myelination after spinal cord injury. Exp Neurol 231:247–260
Yamagishi S, Fujitani M, Hata K, Kitajo K, Mimura F, Abe H, Yamashita T (2005) Wallerian degeneration involves Rho/Rho-kinase signaling. J Biol Chem 280:20384–20388
Yamashita K, Kotani Y, Nakajima Y, Shimazawa M, Yoshimura S, Nakashima S, Iwama T, Hara H (2007) Fasudil, a Rho kinase (ROCK) inhibitor, protects against ischemic neuronal damage in vitro and in vivo by acting directly on neurons. Brain Res 1154:215–224
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627
Zhou Y, Su Y, Li B, Liu F, Ryder JW, Wu X, Gonzalez-DeWhitt PA, Gelfanova V, Hale JE, May PC, Paul SM, Ni B (2003) Nonsteroidal anti-inflammatory drugs can lower amyloidogenic Abeta42 by inhibiting Rho. Science 302:1215–1217
Zhou Q, Gensch C, Liao JK (2011) Rho-associated coiled-coil-forming kinases (ROCKs): potential targets for the treatment of atherosclerosis and vascular disease. Trends Pharmacol Sci 32:167–173
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is funded by the German Research Council (DFG, Research Training School, Neuroinflammation No. 1258 & Exc 247), German Ministry of Science and Education, the Berlin-Brandenburg Center for Regenerative Therapies (BCRT, No. 81717034), the International Foundation for Research in Paraplegia, Switzerland (IFP, no. P102) and Wings for Life Spinal Cord Research Foundation, Austria (no. 89830429). Spinal cord injury Reaserch, Department of Experimental Neurology, Charité is Associated Member of the European Multi-center Study about Spinal Cord Injury
Author disclosure statement
S.M.S. is a co-founder of Axerion Therapeutics seeking to develop NgR- and PrP-based therapies.
Rights and permissions
About this article
Cite this article
Kopp, M.A., Liebscher, T., Niedeggen, A. et al. Small-molecule-induced Rho-inhibition: NSAIDs after spinal cord injury. Cell Tissue Res 349, 119–132 (2012). https://doi.org/10.1007/s00441-012-1334-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-012-1334-7