Abstract
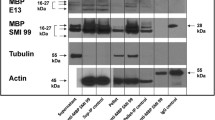
We found colocalization of the neuronal protein p42IP4 (centaurin-α1; ArfGAP with dual pleckstrin homology domain [ADAP1]), the metalloendopeptidase nardilysin (NRD; involved in axonal maturation and myelination) and tubulin in the cytosol and at the plasma membrane of SH-SY5Y neuroblastoma cells. To examine the importance of tubulin for the interaction of NRD with p42IP4, we treated cells with nocodazole, which interferes with tubulin polymerization. Nocodazole did not affect the colocalization of p42IP4 and tubulin but caused a clear redistribution of the proteins in cells, so that the colocalization of p42IP4, tubulin and NRD was visible exclusively in multiple foci. To reveal the mechanism of the interaction between NRD, p42IP4 and tubulin observed in neuronal cells, we performed Far-Western blotting, a technique that directly detects protein-protein interactions on Western blots. This technique demonstrated that tubulin enhanced the binding of NRD to functionally renatured p42IP4. The mutation of a highly conserved cysteine residue in NRD to alanine abolished the potentiation by tubulin. NRD lacking the characteristic acidic domain was able to bind p42IP4 but addition of tubulin did not significantly potentiate the binding of this deletion mutant to p42IP4. A function-abolishing mutation of the Zn2+-binding motif of NRD did not affect the potentiation by tubulin. Thus, the capacity of tubulin to enhance the interaction between p42IP4 and NRD together with the known interaction of p42IP4 with F-actin support the novel notion that p42IP4 plays a possible role as a linker between the two networks, actin and tubulin, in neural cells.





Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- ADAM:
-
A disintegrin and metalloendopeptidase
- Aβ:
-
Amyloid β-peptide
- ADAP:
-
ArfGAP (GTPase activating protein) with dual pleckstrin homology domain
- APP:
-
Amyloid precursor protein
- BACE:
-
β-Secretase
- CNP:
-
2',3'-Cyclic-nucleotide 3'-phosphodiesterase
- DAC:
-
Acidic domain
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FCS:
-
Fetal calf serum
- FSBB:
-
FCS-containing blocking buffer
- GFP:
-
Green fluorescent protein
- HEK293:
-
Human embryonic kidney 293 cells
- NRD1:
-
Nardilysin
- PBS:
-
Phosphate-buffered saline
- PFA:
-
Paraformaldehyde solution
- RanBPM:
-
Ran-binding protein M
- RA:
-
All-trans retinoic acid
- Sf9:
-
Spodoptera frugiperda
- TACE:
-
TNF-α-converting enzyme
- TNF:
-
Tumor necrosis factor
References
Bernstein HG, Stricker R, Dobrowolny H, Trubner K, Bogerts B, Reiser G (2007) Histochemical evidence for wide expression of the metalloendopeptidase nardilysin in human brain neurons. Neuroscience 146:1513–1523
Bernstein HG, Stricker R, Lendeckel U, Bertram I, Dobrowolny H, Steiner J, Bogerts B, Reiser G (2009) Reduced neuronal co-localisation of nardilysin and the putative alpha-secretases ADAM10 and ADAM17 in Alzheimer's disease and Down syndrome brains. Age (Dordr) 31:11–25
Bifulco M, Laezza C, Stingo S, Wolff J (2002) 2',3'-Cyclic nucleotide 3'-phosphodiesterase: a membrane-bound, microtubule-associated protein and membrane anchor for tubulin. Proc Natl Acad Sci USA 99:1807–1812
Bolis A, Coviello S, Visigalli I, Taveggia C, Bachi A, Chishti AH, Hanada T, Quattrini A, Previtali SC, Biffi A, Bolino A (2009) Dlg1, Sec8, and Mtmr2 regulate membrane homeostasis in Schwann cell myelination. J Neurosci 29:8858–8870
Chesneau V, Prat A, Segretain D, Hospital V, Dupaix A, Foulon T, Jegou B, Cohen P (1996) NRD convertase: a putative processing endoprotease associated with the axoneme and the manchette in late spermatids. J Cell Sci 109:2737–2745
Dehmelt L, Halpain S (2007) Neurite outgrowth: a flick of the wrist. Curr Biol 17:R611–R614
Fumagalli P, Accarino M, Egeo A, Scartezzini P, Rappazzo G, Pizzuti A (1998) Human NRD convertase: a highly conserved metalloendopeptidase expressed at specific sites during development and in adult tissues. Genomics 47:238–245
Galvita A, Grachev D, Azarashvili T, Baburina Y, Krestinina O, Stricker R, Reiser G (2009) The brain-specific protein, p42IP4 (ADAP 1) is localized in mitochondria and involved in regulation of mitochondrial Ca2+. J Neurochem 109:1701–1713
Gravel M, Robert F, Kottis V, Gallouzi IE, Pelletier J, Braun PE (2009) 2',3'-Cyclic nucleotide 3'-phosphodiesterase: a novel RNA-binding protein that inhibits protein synthesis. J Neurosci Res 87:1069–1079
Haase A, Nordmann C, Sedehizade F, Borrmann C, Reiser G (2008) RanBPM, a novel interaction partner of the brain-specific protein p42IP4/centaurin α-1. J Neurochem 105:2237–2248
Halpain S, Dehmelt L (2006) The MAP1 family of microtubule-associated proteins. Genome Biol 7:224
Hanck T, Stricker R, Krishna UM, Falck JR, Chang YT, Chung SK, Reiser G (1999) Recombinant p42IP4, a brain-specific 42-kDa high-affinity Ins(1,3,4,5)P4 receptor protein, specifically interacts with lipid membranes containing Ptd-Ins(3,4,5)P3. Eur J Biochem 261:577–584
Hanck T, Stricker R, Sedehizade F, Reiser G (2004) Identification of gene structure and subcellular localization of human centaurin α2, and p42IP4, a family of two highly homologous, Ins 1,3,4,5-P4-/PtdIns 3,4,5-P3-binding, adapter proteins. J Neurochem 88:326–336
Hiraoka Y, Ohno M, Yoshida K, Okawa K, Tomimoto H, Kita T, Nishi E (2007) Enhancement of alpha-secretase cleavage of amyloid precursor protein by a metalloendopeptidase nardilysin. J Neurochem 102:1595–1605
Horiguchi K, Hanada T, Fukui Y, Chishti AH (2006) Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol 174:425–436
Hospital V, Prat A (2004) Nardilysin, a basic residues specific metallopeptidase that mediates cell migration and proliferation. Protein Pept Lett 11:501–508
Kahn RA, Bruford E, Inoue H, Logsdon JM Jr, Nie Z, Premont RT, Randazzo PA, Satake M, Theibert AB, Zapp ML, Cassel D (2008) Consensus nomenclature for the human ArfGAP domain-containing proteins. J Cell Biol 182:1039–1044
Laezza C, Wolff J, Bifulco M (1997) Identification of a 48-kDa prenylated protein that associates with microtubules as 2',3'-cyclic nucleotide 3'-phosphodiesterase in FRTL-5 cells. FEBS Lett 413:260–264
Lakshmana MK, Yoon IS, Chen E, Bianchi E, Koo EH, Kang DE (2009) Novel role of RanBP9 in BACE1 processing of APP and amyloid beta peptide generation. J Biol Chem 284:11863–11872
Ma Z, Csuhai E, Chow KM, Hersh LB (2001) Expression of the acidic stretch of nardilysin as a functional binding domain. Biochemistry 40:9447–9452
Ma Z, Wang X, Hockman S, Snow EC, Hersh LB (2005) Subcellular localization of nardilysin during mouse oocyte maturation. Arch Biochem Biophys 434:187–194
Ohno M, Hiraoka Y, Matsuoka T, Tomimoto H, Takao K, Miyakawa T, Oshima N, Kiyonari H, Kimura T, Kita T, Nishi E (2009) Nardilysin regulates axonal maturation and myelination in the central and peripheral nervous system. Nat Neurosci 12:1506–1513
Pierotti AR, Prat A, Chesneau V, Gaudoux F, Leseney AM, Foulon T, Cohen P (1994) N-arginine dibasic convertase, a metalloendopeptidase as a prototype of a class of processing enzymes. Proc Natl Acad Sci USA 91:6078–6082
Reiser G, Bernstein HG (2002) Neurons and plaques of Alzheimer's disease patients highly express the neuronal membrane docking protein p42IP4/centaurin α. Neuroreport 13:2417–2419
Sedehizade F, Hanck T, Horstmeyer A, Stricker R, Bernstein H-G, Reiser G (2002) Cellular expression and subcellular localization of the human Ins(1,3,4,5)P4-binding protein, p42IP4, in human brain and in neuronal cells. Brain Res Mol Brain Res 99:1–11
Shelanski ML, Gaskin F, Cantor CR (1973) Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci USA 70:765–768
Stricker R, Hülser E, Fischer J, Jarchau T, Walter U, Lottspeich F, Reiser G (1997) cDNA cloning of porcine p42IP4, a membrane-associated and cytosolic 42 kDa inositol(1,3,4,5)tetrakisphosphate receptor from pig brain with similarly high affinity for phosphatidylinositol (3,4,5)P3. FEBS Lett 405:229–236
Stricker R, Adelt S, Vogel G, Reiser G (1999) Translocation between membranes and cytosol of p42IP4, a specific inositol 1,3,4,5-tetrakisphosphate/phosphatidylinositol 3,4,5-trisphosphate-receptor protein from brain, is induced by inositol 1,3,4,5-tetrakisphosphate and regulated by a membrane-associated 5-phosphatase. Eur J Biochem 265:815–824
Stricker R, Chow KM, Walther D, Hanck T, Hersh LB, Reiser G (2006) Interaction of the brain-specific protein p42IP4/centaurin-α1 with the peptidase nardilysin is regulated by the cognate ligands of p42IP4, PtdIns(3,4,5)P3 and Ins(1,3,4,5)P4, with stereospecificity. J Neurochem 98:343–354
Thacker E, Kearns B, Chapman C, Hammond J, Howell A, Theibert A (2004) The arf6 GAP centaurin α-1 is a neuronal actin-binding protein which also functions via GAP-independent activity to regulate the actin cytoskeleton. Eur J Cell Biol 83:541–554
Venkateswarlu K, Hanada T, Chishti AH (2005) Centaurin-α1 interacts directly with kinesin motor protein KIF13B. J Cell Sci 118:2471–2484
Willem M, Garratt AN, Novak B, Citron M, Kaufmann S, Rittger A, DeStrooper B, Saftig P, Birchmeier C, Haass C (2006) Control of peripheral nerve myelination by the beta-secretase BACE1. Science 314:664–666
Wu Y, Li Q, Chen XZ (2007) Detecting protein-protein interactions by Far Western blotting. Nat Protoc 2:3278–3284
Acknowledgements
We are grateful to M. Aswendt for help with the cloning of hsNRD1, to Dr. A. Schneider for help with confocal microscopy, to Dr. T. Hanck for the plasmid pEGFP-p42-C1 and to Dr. S. Aleshin for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was supported by the German federal state of Sachsen-Anhalt within the Europäischer Fond für regionale Entwicklung (EFRE 2007–2013).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Influence of treatment with nocodazole on the morphology of SH-SY5Y cells and localization of NRD (red), p42IP4 (green) and tubulin (blue) within these cells. SH-SY5Y cells were stably transfected with p42IP4-GFP, seeded on coverslips and treated with 10 μM retinoic acid (RA) for 6 days. a Cells were fixed and permeabilized. b Cells were treated with 5 μM nocodazole for 1 h at 37°C before they were fixed and permeabilized for immuncytochemistry. Cells were double-stained with NRD and β-tubulin antibodies and with Alexa Fluor 555, followed by Alexa Fluor 633 antiserum (arrangement of individual images in a as in b). (PDF 136 kb)
Fig. S2
Localization of NRD, p42IP4 and tubulin in SH-SY5Y cells treated with nocodazole. This supplementary figure corresponds to Fig. 2a in the main text. It gives the single confocal images of p42IP4-GFP (green, top left), β-tubulin (blue, bottom left) and NRD (red, top right) in order to complement the merged image. The resulting merged image (bottom right) is identical to Fig. 2a. Conditions for analysis and immuncytochemistry of SH-SY5Y cells stably transfected with p42IP4-GFP are described in the legend of Fig. 2. (PDF 272 kb)
Rights and permissions
About this article
Cite this article
Borrmann, C., Stricker, R. & Reiser, G. Tubulin potentiates the interaction of the metalloendopeptidase nardilysin with the neuronal scaffold protein p42IP4/centaurin-α1 (ADAP1). Cell Tissue Res 346, 89–98 (2011). https://doi.org/10.1007/s00441-011-1245-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1245-z