Abstract
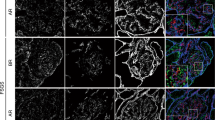
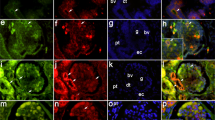
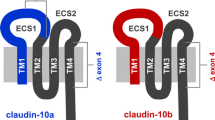
Tight junctions are the main intercellular junctions of podocytes of the renal glomerulus under nephrotic conditions. Their requisite components, claudins, still remain to be identified. We have measured the mRNA levels of claudin subtypes by quantitative real-time PCR using isolated rat glomeruli. Claudin-5 was found to be expressed most abundantly in glomeruli. Mass spectrometric analysis of membrane preparation from isolated glomeruli also confirmed only claudin-5 expression without any detection of other claudin subtypes. In situ hybridization and immunolocalization studies revealed that claudin-5 was localized mainly in glomeruli where podocytes were the only cells expressing claudin-5. Claudin-5 protein was observed on the entire surface of podocytes including apical and basal domains of the plasma membrane in the normal condition and was inclined to be concentrated on tight junctions in puromycin aminonucleoside nephrosis. Total protein levels of claudin-5 in isolated glomeruli were not significantly upregulated in the nephrosis. These findings suggest that claudin-5 is a main claudin expressed in podocytes and that the formation of tight junctions in the nephrosis may be due to local recruitment of claudin-5 rather than due to total upregulation of the claudin protein levels.








Similar content being viewed by others
References
Angelow S, Ahlstrom R, Yu AS (2008) Biology of claudins. Am J Physiol Renal Physiol 295:F867–F876
Caulfield JP, Reid JJ, Farquhar MG (1976) Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 34:43–59
Doné SC, Takemoto M, He L, Sun Y, Hultenby K, Betsholtz C, Tryggvason K (2008) Nephrin is involved in podocyte maturation but not survival during glomerular development. Kidney Int 73:697–704
Furuse M, Tsukita S (2006) Claudins in occluding junctions of humans and flies. Trends Cell Biol 16:181–188
Hirabayashi S, Mori H, Kansaku A, Kurihara H, Sakai T, Shimizu F, Kawachi H, Hata Y (2005) MAGI-1 is a component of the glomerular slit diaphragm that is tightly associated with nephrin. Lab Invest 85:1528–1543
Katayama H, Nagasu T, Oda Y (2001) Improvement of in-gel digestion protocol for peptide mass fingerprinting by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 15:1416–1421
Kerjaschki D (1978) Polycation-induced dislocation of slit diaphragms and formation of cell junctions in rat glomeruli: the effects of low temperature, divalent cations, colchicine, and cytochalasin B. Lab Invest 39:430–440
Kojima S, Rahner C, Peng S, Rizzolo LJ (2002) Claudin 5 is transiently expressed during the development of the retinal pigment epithelium. J Membr Biol 186:81–88
Krause G, Winkler L, Mueller SL, Haseloff RF, Piontek J, Blasig IE (2008) Structure and function of claudins. Biochim Biophys Acta 1778:631–645
Kurihara H, Anderson JM, Kerjaschki D, Farquhar MG (1992) The altered glomerular filtration slits seen in puromycin aminonucleoside nephrosis and protamine sulfate-treated rats contain the tight junction protein ZO-1. Am J Pathol 141:805–816
Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E (1995) The molecular organization of endothelial cell to cell junctionsdifferential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129:203–217
Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660
Peterson GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Pricam C, Humbert F, Perrelet A, Amherdt M, Orci L (1975) Intercellular junctions in podocytes of the nephrotic glomerulus as seen with freeze-fracture. Lab Invest 33:209–218
Rahner C, Mitic LL, Anderson JM (2001) Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology 120:411–422
Reiser J, Kriz W, Kretzler M, Mundel P (2000) The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol 11:1–8
Rodewald R, Karnovsky MJ (1974) Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol 60:423–433
Ryan GB, Leventhal M, Karnovsky MJ (1975) A freeze-fracture study of the junctions between glomerular epithelial cells in aminonucleoside nephrosis. Lab Invest 32:397–403
Sakai N, Chiba H, Fujita H, Akashi Y, Osanai M, Kojima T, Sawada N (2007) Expression patterns of claudin family of tight-junction proteins in the mouse prostate. Histochem Cell Biol 127:457–462
Schnabel E, Dekan G, Miettinen A, Farquhar MG (1989) Biogenesis of podocalyxin–the major glomerular sialoglycoprotein–in the newborn rat kidney. Eur J Cell Biol 48:313–326
Schnabel E, Anderson JM, Farquhar MG (1990) The tight junction protein ZO-1 is concentrated along slit diaphragms of the glomerular epithelium. J Cell Biol 111:1255–1263
Seiler MW, Rennke HG, Venkatachalam MA, Cotran RS (1977) Pathogenesis of polycation-induced alterations ("fusion") of glomerular epithelium. Lab Invest 36:48–61
Shono A, Tsukaguchi H, Yaoita E, Nameta M, Kurihara H, Qin XS, Yamamoto T, Doi T (2007) Podocin participates in the assembly of tight junctions between foot processes in nephrotic podocytes. J Am Soc Nephro 18:2525–2533
Tsukita S, Yamazaki Y, Katsuno T, Tamura A, Tsukita S (2008) Tight junction-based epithelial microenvironment and cell proliferation. Oncogene 27:6930–6938
Usui J, Kurihara H, Shu Y, Tomari S, Kanemoto K, Koyama A, Sakai T, Takahashi T, Nagata M (2003) Localization of intercellular adherens junction protein p120 catenin during podocyte differentiation. Anat Embryol Berl 206:175–184
Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M (2003) Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol 29:62–70
Yaoita E, Yamamoto T, Saito M, Kawasaki K, Kihara I (1991) Desmin-positive epithelial cells outgrowing from rat encapsulated glomeruli. Eur J Cell Biol 54:140–149
Yaoita E, Sato N, Yoshida Y, Nameta M, Yamamoto T (2002a) Cadherin and catenin staining in podocytes in development and puromycin aminonucleoside nephrosis. Nephrol Dial Transplant 17(Suppl 9):16–19
Yaoita E, Yao J, Yoshida Y, Morioka T, Nameta M, Takata T, Kamiie J, Fujinaka H, Oite T, Yamamoto T (2002b) Up-regulation of connexin43 in glomerular podocytes in response to injury. Am J Pathol 161:1597–1606
Zhao L, Yaoita E, Nameta M, Zhang Y, Cuellar LM, Fujinaka H, Xu B, Yoshida Y, Hatakeyama K, Yamamoto T (2008) Claudin-6 localized in tight junctions of rat podocytes. Am J Physiol Regul Integr Comp Physiol 294:R1856–R1862
Zhu Y, Brännström M, Janson PO, Sundfeldt K (2006) Differences in expression patterns of the tight junction proteins, claudin 1, 3, 4 and 5, in human ovarian surface epithelium as compared to epithelia in inclusion cysts and epithelial ovarian tumours. Int J Cancer 118:1884–1891
Acknowledgments
The authors thank Ms. Kanako Oda for her skillful assistance of transplantation of fertilized eggs and Dr. Kosei Takeuchi for helpful discussion and helping transfection of claudins to COS7 cells. This work was supported in part by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology (No. 21591021 to E.Y.).
Author information
Authors and Affiliations
Corresponding author
Additional information
R. Koda and L. Zhao contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Claudin mRNA levels in rat isolated glomeruli quantified by real-time PCR using another primer sets. Quantification was performed with vectors containing each PCR product as a standard. Results are shown as the ratio of claudin to GAPDH, and expressed as the mean +/- SD (n = 4).
Supplementary Fig. S2
Light microscopy (a-d) and electron microscopy (e, f) of glomeruli of neonatal animals from claudin-5 deficient mice (KO) (a, c, e) and wild mice (wild) (b, d, f). Sections for light microscopy were stained with haematoxylin and periodic acid-Schiff reagent (a-d). No morphological difference was found between deficient and wild mice. Pod: podocyte, *: glomerular capillary lumen (GIF 530 kb)
Supplementary Fig. S3
Double-labeled immunofluorescence photomicrographs of frozen kidney sections of neonatal animals from claudin-5 deficient mice (KO) (a, a’) and wild mice (wild) (b, b’) incubated with antibodies against ZO-1 (red; a, b) and podocin (green; a’, b’) (GIF 94 kb)
Supplementary Fig. S4
Double-labeled immunofluorescence photomicrographs of frozen sections of rat neonatal kidneys incubated with antibodies against claudin-5 (green; a, b) and podocin (red; a’) or laminin (red; b). The neonatal kidney displays a developmental gradient with immature glomeruli located toward the kidney surface and more mature glomeruli toward the corticomedullary junction. Staining for claudin-5 is observed in the area closer to the kidney surface but not for podocin (a, a’). Both claudin-5 and podocin are detected in deeper glomeruli of the capillary-loop stage. Double labeling with anti-laminin antibody shows claudin-5 expression at the vesicle stage (b). Dotted lines: kidney surface, v: renal vesicle (GIF 127 kb)
Rights and permissions
About this article
Cite this article
Koda, R., Zhao, L., Yaoita, E. et al. Novel expression of claudin-5 in glomerular podocytes. Cell Tissue Res 343, 637–648 (2011). https://doi.org/10.1007/s00441-010-1117-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-010-1117-y