Abstract
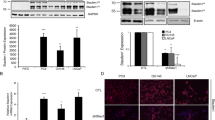
Transgelin is an actin-binding protein shown to be tumour-suppressive. Loss of transgelin expression in transformed cells is associated with oncogenesis. This study aimed to determine whether transgelin expression was suppressed in prostate cancer. An in silico meta-analysis with public-domain expressed-sequence-tag libraries of normal human prostate epithelium, prostatic intraepithelial neoplasia, invasive carcinoma and metastasised lesions predicted decreased transgelin expression with disease progression. Similarly, analysis of Affymetrix gene chip data and the Oncomine database indicated that transgelin was one the 2% most significant of all down-regulated genes in response to prostate cancer. Analysis by quantitative reverse transcription with the polymerase chain reaction (qRT-PCR) of patient biopsies determined transgelin expression to be significantly lower in prostate tumour tissue than in matched normal tissue. Similarly, qRT-PCR and Western blot analysis of representative prostate cancer cell lines demonstrated significantly lower levels of transgelin mRNA and protein in all but the DU145 prostate cancer cell line. Increased expression of TAGLN and increased transgelin protein in response to treatment with transforming growth factor-β suggested that reduced expression in prostate cancer was not attributable to gene promoter suppression by hypermethylation. Gene ontology function analysis highlighted the importance of transgelin in the co-deregulation of actin-binding proteins. Thus, transgelin is suppressed during prostate cancer progression and seems to be an important factor in the dysregulation of the actin cytoskeleton.







Similar content being viewed by others
References
Adam PJ, Regan CP, Hautmann MB, Ownes GK (2000) Positive and negative-acting Kruppel-like transcription factors bind a transforming factor β control element required for expression of smooth muscle cell differentiation marker SM22α in vivo. J Biol Chem 275:37798–37806
Assinder SJ, Stanton J, Prasad PD (2009) Transgelin: an actin binding protein and tumour suppressor. Int J Biochem Cell Biol 41:482–486
Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7:989–995
Bakin AV, Safina A, Rinehart C, Daroqui C, Darbary H, Helfman DM (2004) A critical role of tropomyosins in TGF-β regulation of the actin cytoskeleton and cell motility in epithelial cells. Mol Biol Cell 15:4682–4694
Bar-Sagi D, Hall A (2000) Ras and Rho GTPases: a family reunion. Cell 103:227–238
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubitsta M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Witter CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time experiments. Clin Chem 55:611–622
Button E, Shapland C, Lawson D (1995) Actin, its associated proteins and metastasis. Cell Motil Cytoskeleton 30:247–251
Chandran UR, Ma C, Dhir R, Bisceglia, Lyons-Weiler M, Liang W, Michalopoulos G, Beich M, Monzon FA (2007) Gene expression profiles of prostate cancer reveal involvement of multiple molecular pathways in the metastatic process. BMC Cancer 7:64
Chang JW, Jeon HB, Lee JH, Yoo JS, Chin JS, Kim JH, Yoo YM (2001) Augmented expression of peroxiredoxin I in lung cancer. Biochem Biophys Res Commun 289:507–512
Chen S, Kulik M, Lechleider RJ (2003) Smad proteins regulate transcriptional induction of the SM22α gene by TGF-β. Nucl Acid Res 31:1302–1310
Edgar R, Domrachev M, Lash AE (2002) Gene expression omnibus: NCBI gene expression and hybridisation array data repository. Nucleic Acid Res 30:207–210
Guo Y, Kyprianou N (1999) Restoration of transforming growth factor β signalling pathway in human prostate cancer cells suppresses tumorigenicity via induction of caspase-1-mediated apoptosis. Cancer Res 59:1366–1371
Hirasawa Y, Arai M, Imazeki F, Tada M, Mikata R, Fukai K, Miyazaki M, Ochiai T, Saisho H, Yokosuka O (2006) Methylation status of genes upregulated by demethylating agent 5-aza-2′-deoxycytidine in hepatocellular carcinoma. Oncology 71:77–85
King KJ, Nicholson HD, Assinder SJ (2006) Effect of increasing ratio of estrogen:androgen on proliferation of normal human prostate stromal and epithelial cells, and the malignant cell line LNCaP. Prostate 66:105–114
Lapointe J, Li C, Higgins JP, Rijn M van de, Bair E, Montgomery K, Ferrari M, Egevad L, Rayford W, Bergerheim U, Ekman P, DeMarzo AM, Tibshirani R, Botstein D, Brown PO, Brooks JD, Pollack JR (2004) Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc Natl Acad Sci USA 101:811–818
LaTulippe ESJ, Smith A, Scher H, Scardino P, Reuter V, Gerald WL (2002) Comprehensive gene expression of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res 62:4499–4506
Lawson D, Harris M, Shapland C (1997) Fibroblast transgelin and smooth muscle SM22α are the same protein, the expression of which is down regulated in many cell lines. Cell Motil Cytoskeleton 38:250–257
Leavitt JP, Gunning P, Kedes L, Jarawalla R (1985) Smooth muscle actin is a transformation sensitive marker for mouse NIH 3T3 and Rat-2 cells. Nature 316:840–842
Nair RJ, Solway L, Boyd DD (2006) Expression cloning identifies transgelin (SM22) as a novel repressor of 92-KDa type IV collagenase (MMP-9) expression. J Biol Chem 281:26424–26436
Pawlak G, Helfman DM (2001) Cytoskelatal changes in cell transformation and tumorigenesis. Curr Opin Gene Dev 11:41–47
Peehl DM (2005) Primary cell cultures as models of prostate cancer development. Endocr Relat Cancer 12:19–47
Rhodes DR, Kalyana_Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, Barrette TR, Anstet MJ, Kincead-Beal C, Kulkarni P, Varambally S, Ghosh D, Chinnaiyan AM (2007) Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia 9:166–180
Shapland C, Hsuan JJ, Totty NF, Lawson D (1993) Purification of transgelin: a transformation and shape change sensitive actin-gelling protein. J Cell Biol 121:1065–1073
Shields JM, Rogers-Graham K, Der CJ (2002) Loss of transgelin in breast and colon tumours and in RIE-1 cells by Ras deregulation of gene expression through raf independent pathways. J Biol Chem 277:9790–9799
Sokal RR, Rohlf FJ (1981) Biometry, 2nd edn. Freeman, New York
Stanton JL, Macgregor AB, Green DPL (2002) Using expressed sequence tag databases to identify ovarian genes of interest. Mol Cell Endocrinol 191:11–14
Van Troys M, Vandekerckhove J, Ampe C (2008) Actin and actin-binding proteins in cancer progression and metastasis. In: Remedios C dos, Chhabra D (eds) Protein reviews, vol 8; actin-binding proteins and disease. Springer, New York, pp 229–277
Vanaja DK, Cheville JC, Iturria SJ, Young CYF (2003) Transcriptional silencing of zinc finger protein 185 identified by expression profiling is associated with prostate cancer progression. Cancer Res 63:3877–3882
Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, Wei JT, Pienta KJ, Ghosh D, Rubin MA, Chinnaiyan AM (2005) Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 8:393–406
Varga AE, Stourman NV, Zheng Q, Safina AF, Quan L, Li X, Sossey-Alaoui K, Bakin AV (2005) Silencing of the tropomyosin-1 gene by DNA methylation alters tumor suppressor function of TGF-β. Oncogene 24:5043–5052
Wulfkuhle JD, Sgroi DC, Krutsch H, Maclean K, McGarvey K, Knowlton M, Chen S, Shu H, Sahin A, Kurek R, Wallwiener D, Merino MJ, Petricoin EF III, Zhao Y, Steeg PS (2002) Proteomics of human breast ductal carcinoma in situ. Cancer Res 62:6740–6749
Yang ZM, Chang YJ, Miyamoto H, Ni J, Niu YJ, Chen ZD, Chen YL, Yao JL, Sant’Agnes PA di, Achng CS (2007) Transgelin functions as a suppressor via inhibition of ARA54-enhanced androgen receptor-transactivation and prostate cancer cell growth. Mol Endocrinol 21:343–358
Yu YP, Landsittel D, Jing L, Nelson J, Ren B, Liu L, McDonald C, Thomas R, Dhir R, Finkelstein S, Michalopoulos G, Becich M, Luo J-H (2004) Gene expression alterations in prostate cancer predicting tumor aggression and preceding development of malignancy. J Clin Oncol 22:2790–2799
Zhao L, Wang H, Deng YJ, Wang S, Liu C, Jin H, Ding YQ (2009) Transgelin as a suppressor is associated with poor prognosis in colorectal carcinoma patients. Mod Pathol 22:786–796
Acknowledgements
The authors are grateful to Andrew Macgregor and Chris Mason for technical assistance, to Pacific Edge Biotechnology for analysis of patient samples and to Dr. Alex Tickle of Otago Innovation for her continuing support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Meta-analysis and patient tissue screening was funded by “ART and stem cell markers”, New Economy Research Fund, Foundation for Research, Science and Technology, New Zealand. The authors thank the H.S. and J.C. Anderson Trust, New Zealand for additional financial support. Priya Prasad was supported by a Maori and Pacific Island PhD Scholarship, University of Otago.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPT 563 kb)
Rights and permissions
About this article
Cite this article
Prasad, P.D., Stanton, JA.L. & Assinder, S.J. Expression of the actin-associated protein transgelin (SM22) is decreased in prostate cancer. Cell Tissue Res 339, 337–347 (2010). https://doi.org/10.1007/s00441-009-0902-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0902-y