Abstract
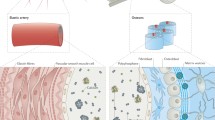
Extracellular matrices (ECM) not only serve as structural scaffolds in organs and tissues, but also determine critical cellular functions through cell-matrix interactions. These are mediated by cell surface receptors that recognise specific structural features of ECMs and, hence, overall physical properties of the acellular environment. ECM structures are subject to hierarchic organisations, which are tightly adapted to the functions of tissues and organs. Only a few specialised tasks are reserved for isolated ECM macromolecules. Instead, molecular ECM components attain their prominent functions only after polymerising into insoluble suprastructural elements, i.e. fibrils, microfibrils, or networks that, in turn, are assembled into regional tissue structures, such as fibres or basement membranes. As an outstanding feature, most, if not all, ECM suprastructures are co-polymers of more than one molecular species that differ in their identity and relative abundance. Thus, ECM suprastructures are composite biological amalgamates. The analogy to metal alloys refers to structural and functional characteristics of ECM composites, which differ from those of each homo-polymeric aggregate. At the tissue level, biological alloys can themselves be assembled into conglomerates that again assume properties distinct from those of each individual alloy. Nevertheless, most studies in matrix biology solely focus on molecular features and mechanisms. Progress has however been made in identifying principles of interactions within suprastructural elements and their functional consequences. We are now only beginning to understand the impact of suprastructural organisation on the assembly and the functions of whole tissues and many fundamental issues in this almost pristine field await discovery.




Similar content being viewed by others
References
Adachi E, Hayashi T (1986) In vitro formation of hybrid fibrils of type V collagen and type I collagen. Limited growth of type I collagen into thick fibrils by type V collagen. Connect Tissue Res 14:257–266
Aumailley M, Battaglia C, Mayer U, Reinhardt D, Nischt R, Timpl R, Fox JW (1993) Nidogen mediates the formation of ternary complexes of basement-membrane components. Kidney Int 43:7–12
Aumailley M, Bruckner-Tuderman L, Carter WG, Deutzmann R, Edgar D, Ekblom P, Engel J, Engvall E, Hohenester E, Jones JCR, Kleinman HK, Marinkovich MP, Martin GR, Mayer U, Meneguzzi G, Miner JH, Miyazaki K, Patarroyo M, Paulsson M, Quaranta V, Sanes JR, Sasaki T, Sekiguchi K, Sorokin LM, Talts JF, Tryggvason K, Uitto J, Virtanen I, Mark K von der, Wewer UM, Yamada Y, Yurchenco PD (2005) A simplified laminin nomenclature. Matrix Biol 24:326–332
Avraamides CJ, Garmy-Susini B, Varner JA (2008) Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer 8:604–617
Baneyx G, Baugh L, Vogel V (2001) Coexisting conformations of fibronectin in cell culture imaged using fluorescence resonance energy transfer. Proc Natl Acad Sci USA 98:14464–14468
Baneyx G, Baugh L, Vogel V (2002) Fibronectin extension and unfolding within cell matrix fibrils controlled by cytoskeletal tension. Proc Natl Acad Sci USA 99:5139–5143
Benninghoff A (1922) Über den funktionellen Bau des Knorpels. Ann Anat 55:250–267
Benninghoff A (1925) Form und Bau der Gelenkknorpel in ihren Beziehungen zur Funktion. II. Der Aufbau des Gelenkknorpels in seinen Beziehungen zur Funktion. Cell Tissue Res 2:783–862
Birk DE, Fitch JM, Babiarz JP, Doane KJ, Linsenmayer TF (1990) Collagen fibrillogenesis in vitro. Interaction of type I and type V collagen regulates fibril diameter. J Cell Sci 95:649–657
Blaschke UK, Eikenberry EF, Hulmes DJS, Galla HJ, Bruckner P (2000) Collagen XI nucleates assembly and limits lateral growth of cartilage fibrils. J Biol Chem 275:10370–10378
Blumbach K, Niehoff A, Paulsson M, Zaucke F (2008) Ablation of collagen IX and COMP disrupts epiphyseal cartilage architecture. Matrix Biol 27:306–318
Brittingham R, Uitto J, Fertala A (2006) High-affinity binding of the NC1 domain of collagen VII to laminin 5 and collagen IV. Biochem Biophys Res Commun 343:692–699
Budde B, Blumbach K, Ylostalo J, Zaucke F, Ehlen HWA, Wagener R, Ala-Kokko L, Paulsson M, Bruckner P, Grässel S (2005) Altered integration of matrilin-3 into cartilage extracellular matrix in the absence of collagen IX. Mol Cell Biol 25:10465–10478
Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE (2004) Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol 165:553–563
Chakravarti S, Petroll WM, Hassell JR, Jester JV, Lass JH, Paul J, Birk DE (2000) Corneal opacity in lumican-null mice: defects in collagen fibril structure and packing in the posterior stroma. Invest Ophthalmol Vis Sci 41:3365–3373
Chan FL, Inoue S (1994) Lamina lucida of basement membrane: an artefact. Microsc Res Tech 28:48–59
Chen H, Mosher DF (1996) Formation of sodium dodecyl sulfate-stable fibronectin multimers. J Biol Chem 271:9084–9089
Cheng Y-S, Champliaud M-F, Burgeson RE, Marinkovich MP, Yurchenco PD (1997) Self-assembly of laminin isoforms. J Biol Chem 272:31525–31532
Cheresh DA, Stupack DG (2008) Regulation of angiogenesis: apoptotic cues from the ECM. Oncogene 27:6285–6298
Clark RAF (2008) Synergistic signaling from extracellular matrix-growth factor complexes. J Invest Dermatol 128:1354–1355
Cukierman E, Pankov R, Stevens DR, Yamada KM (2001) Taking cell-matrix adhesions to the third dimension. Science 294:1708–1712
Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136:729–743
Doane KJ, Birk DE (1991) Fibroblasts retain their tissue phenotype when grown in three-dimensional collagen gels. Exp Cell Res 195:432–442
Dreier R, Opolka A, Grifka J, Bruckner P, Grässel S (2008) Collagen IX-deficiency seriously compromises growth cartilage development in mice. Matrix Biol 27:319–329
Eikenberry EF, Childs B, Sheren SB, Parry DAD, Craig AS, Brodsky B (1984) Crystalline fibril structure of type II collagen in lamprey notochord sheath. J Mol Biol 176:261–277
Ekblom P, Ekblom M, Fecker L, Klein G, Zhang HY, Kadoya Y, Chu ML, Mayer U, Timpl R (1994) Role of mesenchymal nidogen for epithelial morphogenesis in vitro. Development 120:2003–2014
Engler AJ, Humbert PO, Wehrle-Haller B, Weaver VM (2009) Multiscale modeling of form and function. Science 324:208–212
Erickson HP, Carrell NA (1983) Fibronectin in extended and compact conformations; electron microscopy and sedimentation analysis. J Biol Chem 258:14539–14544
Erickson H, Carrell N, McDonagh J (1981) Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J Cell Biol 91:673–678
Farjanel J, Schürmann G, Bruckner P (2001) Contacts with fibrils containing collagen I, but not collagens II, IX and XI, can destabilize the cartilage phenotype of chondrocytes. Osteoarthritis Cartilage 9:S55–S63
Franchi M, Raspanti M, Dell'Orbo C, Quaranta M, De Pasquale V, Ottani V, Ruggeri A (2008) Different crimp patterns in collagen fibrils relate to the subfibrillar arrangement. Connect Tissue Res 49:85–91
Franchi M, Quaranta M, Macciocca M, De Pasquale V, Ottani V, Ruggeri A (2009) Structure relates to elastic recoil and functional role in quadriceps tendon and patellar ligament. Micron 40:370–377
Funakoshi T, Schmid T, Hsu H-P, Spector M (2008) Lubricin distribution in the goat infraspinatus tendon: a basis for interfascicular lubrication. J Bone Joint Surg Am 90:803–814
Gathercole LJ, Keller A (1991) Crimp morphology in the fibre-forming collagens. Matrix Biol 11:214–234
Gendelman R, Burton-Wurster NI, MacLeod JN, Lust G (2003) The cartilage-specific fibronectin isoform has a high affinity binding site for the small proteoglycan decorin. J Biol Chem 278:11175–11181
Grynpas MD, Eyre DR, Kirschner DA (1980) Collagen type II differs from type I in native molecular packing. Biochim Biophys Acta 626:346–355
Henkel W, Glanville RW (1982) Covalent crosslinking between molecules of type I and type III collagen. The involvment of the N-terminal, nonhelical regions of α1 (I) and α1 (III) chains in the formation of intermolecular crosslinks. FEBS J 122:205–213
Holmes DF, Kadler KE (2006) The 10+4 microfibril structure of thin cartilage fibrils. Proc Natl Acad Sci USA 103:17249–17254
Hudson BG, Tryggvason K, Sundaramoorthy M, Neilson EG (2003) Alport's syndrome, Goodpasture's syndrome, and type IV collagen. N Engl J Med 348:2543–2556
Hulmes DJS, Miller A (1979) Quasi-hexagonal packing in collagen fibrils. Nature 282:878–880
Hulmes DJS, Wess TJ, Prockop DJ, Fratzl P (1995) Radial packing, order, and disorder in collagen fibrils. Biophys J 68:1661–1670
Humphries SM, Lu Y, Canty EG, Kadler KE (2008) Active negative control of collagen fibrillogenesis in vivo: intracellular cleavage of the type I procollagen propeptides in tendon fibroblasts without intracellular fibrils. J Biol Chem 283:12129–12135
Hunziker EB, Michel M, Studer D (1997) Ultrastructure of adult human articular cartilage matrix after cryotechnical processing. Microsc Res Tech 37:271–284
Järvinen TAH, Järvinen TLN, Kannus P, Józsa L, Järvinen M (2004) Collagen fibres of the spontaneously ruptured human tendons display decreased thickness and crimp angle. J Orthop Res 22:1303–1309
Johnson KJ, Sage H, Briscoe G, Erickson HP (1999) The compact conformation of fibronectin is determined by intramolecular ionic interactions. J Biol Chem 274:15473–15479
Kadler KE, Hill A, Canty-Laird EG (2008) Collagen fibrillogenesis: fibronectin, integrins, and minor collagens as organizers and nucleators: cell-to-cell contact and extracellular matrix. Curr Opin Cell Biol 20:495–501
Kadoya Y, Salmivirta K, Talts JF, Kadoya K, Mayer U, Timpl R, Ekblom P (1997) Importance of nidogen binding to laminin γ1 for branching epithelial morphogenesis of the submandibular gland. Development 124:683–691
Keene DR, Sakai LY, Lunstrum GP, Morris NP, Burgeson RE (1987) Type VII collagen forms an extended network of anchoring fibrils. J Cell Biol 104:611–621
Korpos E, Wu C, Sorokin L (2009) Multiple roles of the extracellular matrix in inflammation. Curr Pharm Des 15:1349–1357
Koyama H, Raines EW, Bornfeldt KE, Roberts JM, Ross R (1996) Fibrillar collagen inhibits arterial smooth muscle proliferation through regulation of Cdk2 inhibitors. Cell 87:1069–1078
Lahav J, Lawler J, Gimbrone MA (1984) Thrombospondin interactions with fibronectin and fibrinogen. FEBS J 145:151–156
Leiss M, Beckmann K, Girós A, Costell M, Fässler R (2008) The role of integrin binding sites in fibronectin matrix assembly in vivo: cell-to-cell contact and extracellular matrix. Curr Opin Cell Biol 20:502–507
Mao Y, Schwarzbauer JE (2005) Fibronectin fibrillogenesis, a cell-mediated matrix assembly process. Matrix Biol 24:389–399
Mayer U, Nischt R, Pöschl E, Mann K, Fukuda K, Gerl M, Yamada Y, Timpl R (1993) A single EGF-like motif of laminin is responsible for high-affinity nidogen binding. EMBO J 12:1879–1885
Mayer U, Zimmermann K, Mann K, Reinhardt D, Timpl R, Nischt R (1995) Binding-properties and protease stability of recombinant human nidogen. FEBS J 227:681–686
Mayer U, Mann K, Fessler LI, Fessler JH, Timpl R (1997) Drosophila laminin binds to mammalian nidogen and to heparan sulfate proteoglycan. FEBS J 245:745–750
Mendler M, Eich-Bender SG, Vaughan L, Winterhalter KH, Bruckner P (1989) Cartilage contains mixed fibrils of collagen types II, IX, and XI. J Cell Biol 108:191–197
Minns RJ, Steven FS (1977) The collagen fibril organization in human articular cartilage. J Anat 123:437–457
Mokkapati S, Baranowsky A, Mirancea N, Smyth N, Breitkreutz D, Nischt R (2008) Basement membranes in skin are differently affected by lack of nidogen 1 and 2. J Invest Dermatol 128:2259–2267
Nishimichi N, Higashikawa F, Kinoh HH, Tateishi Y, Matsuda H, Yokosaki Y (2009) Polymeric osteopontin employs integrin α9β1 as a receptor and attracts neutrophils by presenting a de novo binding site. J Biol Chem 284:14769–14776
Ohashi T, Erickson HP (2009) Revisiting the mystery of fibronectin multimers: the fibronectin matrix is composed of fibronectin dimers cross-linked by non-covalent bonds. Matrix Biol 28:170–175
Olsen BR (1963) Electron microscope studies on collagen. II. Mechanism of linear polymerization of tropocollagen molecules. Cell Tissue Res 59:199–213
Orgel JPRO, Irving TC, Miller A, Wess TJ (2006) Microfibrillar structure of type I collagen in situ. Proc Natl Acad Sci USA 103:9001–9005
Petruska JA, Hodge AJ (1964) A subunit model for the tropocollagen macromolecule. Proc Natl Acad Sci USA 51:871–876
Poole CA, Flint MH, Beaumont BW (1987) Chondrons in cartilage: ultrastructural analysis of the pericellular microenvironment in adult human articular cartilages. J Orthop Res 5:509–522
Price RI, Lees S, Kirschner DA (1997) X-ray diffraction analysis of tendon collagen at ambient and cryogenic temperatures: role of hydration. Int J Biol Macromol 20:23–33
Prockop DJ, Kivirikko KI (1995) Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64:403–434
Quantock AJ, Young RD (2008) Development of the corneal stroma, and the collagen-proteoglycan associations that help define its structure and function. Dev Dyn 237:2607–2621
Ruben GC, Yurchenco PD (1994) High resolution platinum-carbon replication of freeze-dried basement membrane. Microsc Res Tech 28:13–28
Schmidt G, Hausser H, Kresse H (1991) Interaction of the small proteoglycan decorin with fibronectin: involvement of the sequence NKISK of the core protein. Biochem J 280:411–414
Scott JE, Thomlinson AM (1998) The structure of interfibrillar proteoglycan bridges (“shape modules”) in extracellular matrix of fibrous connective tissues and their stability in various chemical environments. J Anat 192:391–405
Sechler JL, Schwarzbauer JE (1997) Coordinated regulation of fibronectin fibril assembly and actin stress fiber formation. Cell Adhes Commun 4:413–424
Segawa K, Takiguchi R (1992) Ultrastructural alteration of cartilaginous fibril arrangement in the rat mandibular condyle as revealed by high-resolution scanning electron microscopy. Anat Rec 234:493–499
Sercu S, Zhang M, Oyama N, Hansen U, Ghalbzouri AEL, Jun G, Geentjens K, Zhang L, Merregaert JH (2008) Interaction of extracellular matrix protein 1 with extracellular matrix components: ECM1 is a basement membrane protein of the skin. J Invest Dermatol 128:1397–1408
Sipes J, Guo N, Negre E, Vogel T, Krutzsch H, Roberts D (1993) Inhibition of fibronectin binding and fibronectin-mediated cell adhesion to collagen by a peptide from the second type I repeat of thrombospondin. J Cell Biol 121:469–477
Stolz M, Gottardi R, Raiteri R, Miot S, Martin I, Imer R, Staufer U, Raducanu A, Düggelin M, Baschong W, Daniels AU, Friederich NF, Aszódi A, Aebi U (2009) Early detection of aging cartilage and osteoarthritis in mice and patient samples using atomic force microscopy. Nat Nanotechnol 4:186–192
Strasser S, Zink A, Janko M, Heckl WM, Thalhammer S (2007) Structural investigations on native collagen type I fibrils using AFM. Biochem Biophys Res Commun 354:27–32
Streuli CH (2009) Integrins and cell-fate determination. J Cell Sci 122:171–177
Sylvester MF, Yannas IV, Salzman EW, Forbes MJ (1989) Collagen banded fibril structure and the collagen-platelet reaction. Thromb Res 55:135–148
Timpl R, Wiedemann H, Delden V van, Furthmayr H, Kühn K (1981) A network model for the organization of type IV collagen molecules in basement membranes. FEBS J 120:203–211
Vaughan L, Mendler M, Huber S, Bruckner P, Winterhalter KH, Irwin MI, Mayne R (1988) D-periodic distribution of collagen IX along cartilage fibrils. J Cell Biol 106:991–997
Villone D, Fritsch A, Koch M, Bruckner-Tuderman L, Hansen U, Bruckner P (2008) Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils. J Biol Chem 283:24506–24513
Wachtel E, Maroudas A (1998) The effects of pH and ionic strength on intrafibrillar hydration in articular cartilage. Biochim Biophys Acta 1381:37–48
Wess TJ (2005) Collagen fibril form and function. In: Parry DAD, Squire JM (eds) Advances in protein chemistry: fibrous proteins: coiled-coils, collagen and elastomers. Academic Press, New York, pp 341–374
Wiberg C, Klatt AR, Wagener R, Paulsson M, Bateman JF, Heinegĺrd D, Mörgelin M (2003) Complexes of matrilin-1 and biglycan or decorin connect collagen VI microfibrils to both collagen II and aggrecan. J Biol Chem 278:37698–37704
Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, Timpl R, Mayer U (2002) Specific ablation of the nidogen-binding site in the laminin gamma1 chain interferes with kidney and lung development. Development 129:2711–2722
Wu JJ, Weis MA, Kim LS, Carter BG, Eyre DR (2009) Differences in chain usage and cross-linking specificities of cartilage type V/XI collagen isoforms with age and tissue. J Biol Chem 284:5539–5545
Yurchenco PD, Ruben GC (1987) Basement membrane structure in situ. Evidence for lateral associations in the type IV collagen network. J Cell Biol 105:2559–2568
Yurchenco PD, Ruben GC (1988) Type IV collagen lateral associations in the EHS tumor matrix. Comparison with amniotic and in vitro networks. Am J Pathol 132:278–292
Zhang G, Young BB, Ezura Y, Favata M, Soslowsky LJ, Chakravarti S, Birk DE (2005) Development of tendon structure and function: regulation of collagen fibrillogenesis. J Musculoskelet Neuronal Interact 5:5–21
Zhang G, Chen S, Goldoni S, Calder BW, Simpson HC, Owens RT, McQuillan DJ, Young MF, Iozzo RV, Birk DE (2009) Genetic evidence for the coordinated regulation of collagen fibrillogenesis in the cornea by decorin and biglycan. J Biol Chem 284:8888–8897
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bruckner, P. Suprastructures of extracellular matrices: paradigms of functions controlled by aggregates rather than molecules. Cell Tissue Res 339, 7–18 (2010). https://doi.org/10.1007/s00441-009-0864-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-009-0864-0