Abstract
The lymph node sinus are channel structures of unquestionable importance in immunology and pathology, specifically in the filtering of the lymph, the transport and processing of antigens, the adhesion and migration of immune cells, and the spread of metastatic cancer cells. Our knowledge of the cell and molecular biology of the sinus-forming cells is still limited, and the origin and biological nature of these cells have long been a matter of debate. Here, we review the relevant literature and present our own experimental results, in particular concerning molecular markers of intercellular junctions and cell differentiation. We show that both the monolayer cells lining the sinus walls and the intraluminal virgultar cell meshwork are indeed different morphotypes of the same basic endothelial cell character, as demonstrated by the presence of a distinct spectrum of general and lymphatic endothelial markers, and we therefore refer to these cells as sinus endothelial/virgultar cells (SEVCs). These cells are connected by unique adhering junctions, termed complexus adhaerentes, characterized by the transmembrane glycoprotein VE-cadherin, combined with the desmosomal plaque protein desmoplakin, several adherens junction plaque proteins including α- and β-catenin and p120 catenin, and components of the tight junction ensemble, specifically claudin-5 and JAM-A, and the plaque protein ZO-1. We show that complexus adhaerentes are involved in the tight three-dimensional integration of the virgultar network of SEVC processes along extracellular guidance structures composed of paracrystalline collagen bundle “stays”. Overall, the SEVC system might be considered as a local and specific modification of the general lymphatic vasculature system. Finally, physiological and pathological alterations of the SEVC system will be presented, and the possible value of the molecular markers described in histological diagnoses of autochthonous lymph node tumors will be discussed.
Similar content being viewed by others
Introduction
Most of the vascular system of the mammalian body in which fluid and cells are transported, including both blood and lymph vessels, is composed of endothelium-lined tubes or channels of widely variable caliber, which exhibit a free lumen. In some parts, however, venous and lymphatic vessels occur from which valvular structures project into the lumen thereby contributing to the unidirectional flow. A unique exception to the structural principle of the free lumen in the vasculature is represented by the sinusFootnote 1 of lymph nodes, i.e., the lymph system leading from the afferent vessels via the subcapsular (marginal) and the intermediate (cortical) sinus to the medullary sinus and finally the efferent lymphatic vessels. Light- and electron-microscopic studies have shown that the sinus contain a relatively dense meshwork of cell processes and extracellular fibers (for reviews, see for example, Aschoff 1924; Akazaki 1953; Raviola 1975; Sakuma et al. 1981; Wacker 1994). Thus, the potentially interactive, reactive, and binding surface of the total endothelial cell lining and of the cells forming the luminal meshwork is greatly enhanced. As a result, this meshwork reduces the flow speed of the percolating lymph components considerably. Obviously, the sinus with their outer lining and the intraluminal “virgultum”Footnote 2 system are placed effectively for filtering and interacting with other cells (in particular, for interactions with the various antigen-presenting cells, lymphocytes, and macrophages) and with variously sized particles, complexes, and free molecules (e.g., Drinker et al. 1933, 1934; Forkert et al. 1977; for further references, see below). Specifically, a series of recent reports has demonstrated the importance of the sinus-lining cells and the luminal meshwork, including the trabeculae-ensheathing cell layer, for the sorting, transport, and processing of lymph components (for recent references, see Gretz et al. 2000; von Andrian and Mempel 2003; Prevo et al. 2004; Sixt et al. 2005; Bajénoff et al. 2006). The lymph node sinus system is also known to be of eminent importance in various pathological processes, including immunological diseases and, in particular, cancer cell metastasis. The limited extent of the information concerning the cell and molecular biology of the lymph node sinus system is therefore surprising.
The nature of the surface-lining and of the luminal cells of the lymph node sinus has been a matter of debate for a long time. Whereas the sinus walls are covered by a layer of flat endothelium-like cells, the intraluminal virgultum is composed of partly stellate cells of dendritic morphology, characterized by thin, often branched processes, some of which are closely associated with fine “reticular fibers” containing collagen bundles. In earlier studies (e. g., Akazaki 1953), the outer sinus lining cells were regarded as an endothelium or reticulo-endothelium and the meshwork in the sinus lumina as reticulum cells. On the other hand, Aschoff (1924) had previously suggested that they were essentially two morphotypes of the same cell type system of “reticulo-endothelial cells”, which, on the one hand, lined the sinusal spaces and, on the other hand, formed the intrasinusal meshwork, i.e., the virgultum. Other authors have used the terms “reticular lining cells” and “trabecular reticulum cells” for the sinus-lining cells and the intrasinusal reticular cells, respectively, but have also emphasized their ultrastructural and cell type relationship (e.g., Farr et al. 1980). More recently, however, several authors have argued against a relationship of the sinus-lining cells and the luminal cells to vascular endothelial cells and have denied their endothelial character (e.g., Wacker 1994; Wacker et al. 1997; for discussions of earlier references see therein and, e.g., Forkert et al. 1977). In the present article, molecular biological data from our group and from several other laboratories are presented to demonstrate that (1) sinus-wall-lining cells and intrasinusal meshwork cells belong together essentially as two morphotypes of the same cell type, and (2) both morphotypes represent a distinct subform of a vascular endothelial cell related to, but in certain aspects distinct from, the endothelial cells of lymphatic vessels. For the sake of both simplicity and clarity, we shall collectively refer to these cells as the “sinus endothelial/virgultar cell” (SEVC) system.
In particular, we shall focus on a peculiar structural feature common to the SEVCs and, partly, also to certain other lymphatic endothelial cells, i.e., the specialized and unique cell-cell junctions of the complexus adhaerens type. In 1990, we noticed that variably sized, often extended cell-cell junctional structures connected the SEVCs of the lymph node sinus, not only in the sinus-lining cell layer, but also in the virgultar cell meshwork in the lumina, and that these were a type of adhering junctions sui generis, both with respect to their morphology and their molecular composition (Schmelz et al. 1990; Schmelz and Franke 1993). Not only the cells of the outer lining endothelial cell layer, but also the intrasinusal SEVCs were interconnected by complex adhering junctions characterized by a highly complex and variable morphology and a characteristic VE-cadherin-based junction molecular ensemble, anchored in an α- and β-catenin-containing cytoplasmic plaque that was conspicuously rich in desmoplakin, a protein known to be a marker for epithelial desmosomes and the composite junctions of cardiomyocytes (Franke et al. 1981, 1982, 1994, 2006; Schmelz and Franke 1993; Schmelz et al. 1994; Hämmerling et al. 2006; Borrmann et al. 2006). Similar desmoplakin-positive adhering junctions have also been identified in endothelial cells of certain lymphatic vessels, and desmoplakin also appears to be important in junctional assemblies of certain endothelia in embryogenesis (Gallicano et al. 2001; Zhou et al. 2004; for general aspects, see also Valiron et al. 1996; Kowalczyk et al. 1998; Cattelino et al. 2003; Dejana et al. 1999, 2000; Sleeman et al. 2001; Stacker et al. 2002; Dejana 2004).
Desmoplakin has therefore been introduced as a further special diagnostic marker for a certain part of the lymphatic endothelial system (see, e.g., Ebata et al. 2001a, b), which histochemically is practically defined as a type of vessel positive both for desmoplakin and α- and β-catenin on the one hand (Schmelz et al. 1994; Franke et al. 1994) and for lymphatic markers such as LYVE-1 on the other (e.g., Banerji et al. 1999; for reviews, see also Stacker et al. 2002; Al-Rawi et al. 2005). This novel and specific kind of adhering junction in SEVCs and endothelia of lymphatic vessels has been generally termed the complexus adhaerens, the “complex junction”. The molecular complexity has subsequently turned out to be even greater, as certain molecules of the tight junction ensemble such claudin-5 and zonula occludens protein ZO-1 are also regularly demonstrable within the morphological confinements of the complexus adhaerentes (Hämmerling 2004; Hämmerling et al. 2006; see also Baluk et al. 2007; Pfeiffer et al. 2008). Thus, the emerging picture of the complexus adhaerens seems to be that of an unusually complex combination of junction molecules, combining members of adherens junctions, such as the zonula adhaerens and desmosomes, with those of tight junctions. Most recently, this unusually complex composition has been confirmed and markedly extended by Pfeiffer et al. (2008).
Because of this astonishingly high molecular complexity and the general importance for our understanding of molecular interactions and functions in cell-cell coupling, and in view of the great importance of the SEVC system of lymph nodes in immunology and in cancer cell metastatic processes, we review here accumulated and newly added information and also summarize data on SEVCs in certain “reactive” and pathological states. We also discuss possible special functions of SEVCs and the pathological importance of these molecular markers in lymph node pathology, including the diagnosis of autochthonous tumors of lymph nodes.
Materials and methods
Preparations of snap-frozen human lymph nodes have been previously described (e.g., Schmelz et al. 1994; Hämmerling 2004; Hämmerling et al. 2006; Sievers 2009). Paraffin blocks containing normal and pathologically altered human lymph nodes were taken from the archival files of the Institute of Pathology of the University of Marburg. These samples had been obtained during surgery for diagnostic or therapeutic reasons and had been routinely formaldehyde-fixed immediately after removal and subsequently embedded in paraffin. Some lymph nodes were obtained as fresh tissue samples immediately snap-frozen in liquid nitrogen, representing residual tissue after frozen tissue section diagnosis. All human tissue samples were taken for the study only after pathological diagnosis had been completed and were used without knowledge of the personal data of the patients. Fresh lymph node tissue samples of bovine origin were obtained from the abattoir at Mannheim and fixed as described (see, e.g., Schmelz and Franke 1993; Hämmerling et al. 2006). The preparation of cryostat and paraffin sections and further processing for immunolocalization experiments were as previously reported, including conventional immunofluorescence microscopy and confocal laser-scanning microscopy (single- and double-label) of cryostat sections and avidin-biotin-based immunohistochemistry of sections of paraffin-embedded tissue samples (Schmelz et al. 1994; Hämmerling et al. 2006; Sievers 2009). Tissue samples for conventional and immunolabeling electron microscopy were fixed and processed as previously described (Schmelz et al. 1994; Hämmerling et al. 2006).
Primary antibodies used, their sources, and methodological details, including antigen retrieval procedures for paraffin sections, are listed in Supplementary Table 1 (see also Schmelz and Franke 1993; Schmelz et al. 1994; Borrmann et al. 2006; Hämmerling et al. 2006; Moll et al. 2006; Wuchter et al. 2007). Micrographs of serial sections were analyzed by using tomo- and topographical protocols (Schmelz and Franke 1993).
Results and discussion
Lymph nodes are essential secondary immune organs that are positioned at strategically important sites along the lymphatic system in the body. Soluble antigens, complexes, particles and immune and other cells enter the lymph nodes with the lymph flow via the afferent lymphatic vessels from the respective drainage area. Alternatively, cells may enter lymph nodes by extravasation from postcapillary venules of the blood stream. Whereas considerable information has been accumulated with respect to the structure and organization of the lymph node and its functions in the initiation and coordination of the cellular immune response (for a recent review, see Willard-Mack 2006), relatively little is known about the surface-lining and intraluminal cell structures of the lymph node sinus, the SEVC system.
This cellular system comprises two morphologically different categories of cells: the cells lining the sinus margins and the multiform, partly branched, “stellate” cells forming a meshwork in the sinus lumen. The latter cover the collagen fibril bundle “stays” (supports) of the sinus lumen, forming irregular-appearing virgultar arrays. Both forms of SEVCs are characterized by cytoplasmic projections, frequent cell-cell contacts, and variously sized junctional complexes. The endothelial cell layer of the sinus-lining cells and the three-dimensional virgultar meshwork aspect of the intraluminal cells are also directly visible in surface-scanning electron micrographs (see, e.g., Fujita et al. 1972; Raviola 1975; Farr et al. 1980; Ushiki et al. 1995). On the other hand, this thicket-like cell meshwork organization characterized by numerous cell processes interconnected by variously sized junctions obviously does not meet many researchers’ view of an “endothelium”. Thus, several authors have concluded that these cells, which are among the most frequent cell types in the mature lymph node, are not of a vascular endothelial nature or origin but represent special forms of non-endothelial differentiation, probably of a monocytic type, and should be regarded as “immune accessory cells”, i.e., cells of a three-dimensional system serving accessory immunological functions (for reviews and controversial discussions, see, e.g., Raviola 1975; Crivellato and Mallardi 1998; Wacker 1994; Wacker et al. 1997; Kaldjian et al. 2001; Willard-Mack 2006; see also below).
Indeed, we have learned with some surprise that two different kinds of lymph node cells with three-dimensional meshwork morphology, viz., the follicular dendritic reticulum cells (FDCs) and the SEVCs, have some structural aspects and molecules in common, such as the protein desmoplakin, forming a junction-associated cytoplasmic coat (Schmelz et al. 1990, 1994; Schmelz and Franke 1993; Franke et al. 1994; Wacker 1994; Hämmerling et al. 2006), which so far has only been reported for epithelial, myocardial, and meningeal cells (e.g., Franke et al. 1982; Kartenbeck et al. 1984; Moll et al. 1986; Akat et al. 2008). The idea that the FDCs represent immune accessory cells playing an important functional role in the humoral immune response appears to be generally accepted (e.g., Tew et al. 1990, 2001). However, although the junctional protein desmoplakin is common to both FDCs and SEVCs, the underlying junctional structures and molecular ensembles are very different (see below). Moreover, the SEVCs have also recently been found to be positive, by immunostaining, for an increasing number of endothelial marker molecules including markers of lymphatic endothelial cells (Schmelz and Franke 1993; Schmelz et al. 1994; Hämmerling et al. 2006; Pfeiffer et al. 2008; and data below).
Using a series of cell differentiation markers for immunocytochemistry at the light- and electron-microscopical level, we have therefore systematically characterized the cytoskeletal and junctional proteins and glycoproteins of the SEVC system of lymph nodes in mammals. In particular, we have demonstrated not only the essentially endothelial character of these cells, but also that they are connected by adhering junctions that have a highly complex morphology and a unique molecular composition, and that therefore have been subsumed under the collective name “complexus adhaerens” (Schmelz and Franke 1993). Whereas some of these junctions, notably those of the sinus-lining cells, i.e., endothelial cells sensu stricto, appear predominantly as distinct puncta, fasciae, or zonulae of the adherens junction type, we have mostly focused on the complex, often extended, junctional structures connecting cells of the virgultar meshwork of the SEVCs in the sinus lumina, i.e., structural complexes that have not yet been seen in any other cell type (this category of complex formations has been termed “syndesmos”; Schmelz and Franke 1993). Both the sinus-lining endothelial and intraluminal virgultar cells are characterized by the general presence of highly specialized junctions of the complexus adhaerens type. Moreover, they exhibit essentially identical differentiation marker profiles (see below). Thus, in cell biological terms, they appear to represent one cell type, viz., SEVC, a term that covers both these morphotypes. Moreover, the desmoplakin-containing complexus adhaerentes are a striking feature common to both SEVC morphotypes but also occur in certain other endothelial cells such as in the endothelia of small and medium-sized lymphatic vessels of, e.g., the dermis and the lingual mucosa (Schmelz et al. 1994; Hämmerling 2004; Hämmerling et al. 2006; see also Ebata et al. 2001a, b).
Light and electron microscopy of SEVCs
By light microscopy (for reviews, see, e.g., Raviola 1975; Wacker 1994; Willard-Mack 2006), subcapsular, intermediate, and medullary sinus of mammalian lymph nodes are characterized by a variably dense meshwork of intraluminal dendritic virgultar cells, some of which are, at certain points, connected to the monolayer of sinus-lining endothelial cells (see, e.g., Fig. 1a). The spaces that lie within the intrasinusal virgultum, and that vary in width depending on the functional state of the lymph node, contain lymph fluid and variable numbers of cells, notably macrophages (specifically sinus histiocytes) and lymphocytes. Often the SEVCs are difficult to discern at the light-microscopic level, in particular when the sinus is narrow or when it is filled with lymphocytes or histiocytes. Cytologically, the subtypes of SEVCs are inconspicuous, as they usually possess small nuclei and thin cytoplasmic structures. The nuclei are usually elongated in the sinus-lining endothelial cells but are plumper in the virgultar cells. The cytoplasm of the lining cells is mostly much attenuated, often resulting in a central nucleus-containing protrusion. The cells of the intrasinusal virgultar meshwork are characterized by thin, but often long, branching and anastomosing dendritic cytoplasmic processes, which occasionally may be up to 100 µm long (see also Wacker 1994), and which are connected to each other and to the sinus walls.
Light microscopy and immunohistochemistry of structural proteins of the sinus endothelial/virgultar cell (SEVC) system in human lymph nodes, illustrated by the example of a subcapsular sinus (axillary lymph node, paraffin sections). a–d The capsule (C) lies top above the subcapsular sinus (brackets). a Hematoxylin and eosin (H&E) staining. The intrasinusal SEVC meshwork consists of delicate cells (arrows nuclei of some of these cells) with thin dendritic processes. Some lymphocytes are seen to be included in the virgultar meshwork. b Gomori’s silver impregnation. The fine meshwork of intrasinusal reticulin fibers (arrows) covered by SEVCs and their processes is visualized. Note the continuity of these fine reticulin fibers with the thicker fibers of the reticular meshwork of the adjacent parenchyma (bottom margin). c Immunohistochemistry for collagen IV. Positive staining of intrasinusal fibers (arrows some cross-sectioned fibers) and outline of basement membrane beneath the SEVCs lining the sinus walls. d Cytoplasm of SEVCs is strongly positive for the intermediate filament protein vimentin. Inset (1.7-fold higher magnification): SEVC process (arrow) showing a double contour of vimentin positivity (corresponding to the SEVC process cytoplasm) ensheathing a negative core corresponding to a reticulin fiber. Bar 100 µm (in a)
The free luminal regions in the intrasinusal virgultum are not merely composed of SEVC processes but are structurally enforced by extracellular collagen bundles that can be readily visualized as fine fibrils by Gomori’s silver stain (Fig. 1b; see also Wacker et al. 1997). The fibrilllar network supporting the intrasinusal SEVC meshwork contains collagen type IV, as is also demonstrable by immunohistochemistry (Fig. 1c). With van Gieson’s trichrome stain, however, only a few of the intrasinusal reticular fibers appear with a faint red stain corresponding to collagen (not shown). The three-dimensional organization of the virgultar framework in the lymph node sinus and its association with the adjacent capsule and parenchyma have been demonstrated with particular clarity by scanning electron microscopy, with and without maceration (Ushiki et al. 1995). These studies have also clearly demonstrated continuities of the reticular fiber system from the capsule to the SEVCs and the parenchyma (as already suggested at the light-microscopical level by Gomori’s silver stain, see Fig. 1b), indicating that the virgultar fiber system spanning the sinus can be considered as part of a continuous skeletal framework of the lymph node.
By transmission electron microscopy (e.g., Moe 1963; Forkert et al. 1977; Farr et al. 1980; Sakuma et al. 1981; Compton and Raviola 1985; Schmelz and Franke 1993; Wacker 1994; Wacker et al. 1997; Ushiki et al. 1995; Crivellato and Mallardi 1998), the cytoplasm of SEVCs, particularly the sinus-lining SEVCs, generally appears relatively poor in organelles. Often, these cells contain variable numbers of vesicles, including pinocytotic vesicles and secondary lysosomes (Moe 1963; Forkert et al. 1977; Sakuma et al. 1981). Experiments have shown that SEVCs are able to take up particulate material such as ferritin by phagocytosis (Farr et al. 1980; Compton and Raviola 1985); however, they lack the abundant pleomorphic phagosomes and phagolysosomes characteristic of macrophages (see also Wacker et al. 1997). At least in some species, plasma membrane invaginations closed by a diaphragm (Compton and Raviola 1985) or intracytoplasmic tubulovesicular structures communicating with the sinus lumen (Crivellato and Mallardi 1998) have been described in SEVCs.
Furthermore, the SEVCs contain bundles of intermediate-sized filaments (IFs; Compton and Raviola 1985; Schmelz and Franke 1993; Wacker 1994; see also below), which, in mammals, are exclusively of the vimentin type characteristic of endothelial cells in general (Fig. 1d; Franke et al. 1979, 1988; Schmelz and Franke 1993; for the occurrence of cytokeratin IFs in certain endothelial and other vascular wall cells, see Franke et al. 1989b). These IF bundles mostly show only limited association with the plaques of the special cell-cell junctions (complexus adhaerentes; Schmelz and Franke 1993).
SEVCs are quiescent cells lacking significant proliferative activity. Mitoses are essentially absent (Wacker 1994), and the proliferation marker Ki-67, indicative of cells in the active phases of the cell cycle, is usually detected only in a few parenchyma-lining SEVCs, i.e., approximately in one out of 500 cells (Kaiserling et al. 1998).
Especially impressive morphological features of the intrasinusal SEVCs are their extended, often flat, but broad cytoplasmic processes, which can almost completely ensheath the collagenous bundles by the formation of channel-like plasma-membrane invaginations. By longitudinal closure of the dendritic SEVC processes, which are supported by complexus adhaerentes, tubes are formed whose lumina, in which the fibrils run, belong to the extracellular space (Moe 1963; Crivellato and Mallardi 1998) and are continuous with the extracellular space layer between sinus-lining SEVCs and fibroblastic reticular cells (FRCs; see below). These structures, consisting of SEVC processes and extracellular matrix, represent the virgultum of the lymph node sinus and will be described in more detail in “Electron microscopy of cell-cell connections in the SEVC system”.
Diverse observations have been reported concerning the presence of a basement membrane or basal lamina structure adjacent to the abluminal cell surface of the sinus-lining cells of the SEVC system (see also below). While no such basal lamina has been reported from rat and rabbit lymph node sinus (e.g., Farr et al. 1980; Compton and Raviola 1985), Moe (1963) described the presence of an inconstant basement membrane along the lining SEVCs in mouse lymph nodes. In human lymph nodes, Forkert et al. (1977) have reported the presence of a basal lamina supporting the SEVCs lining the capsule and the trabeculae, whereas an interrupted basal lamina appears to line the sinus on the parenchyma side. No basal lamina has been detected on SEVCs of the medullary sinus. Wacker et al. (1997) have mentioned SEVC dendrites attached to basal membrane structures of the sinus walls. Immunohistochemically, laminin-positive basement membranes have been described to occur along subcapsular sinus, including their walls bordering on the parenchyma (Reilly et al. 1985), and also along the medullary sinus (Pfeiffer et al. 2008).
The SEVCs lining the capsular and trabecular surfaces of the sinus appear to form a continuous unbroken endothelial monolayer (Forkert et al. 1977). Moreover, the inner surfaces of the sinus facing the parenchyma seem to be extensively lined by SEVCs, except for certain intercellular gaps the existence of which is, however, controversially discussed in the literature (cf. Gretz et al. 2000). Whereas Farr et al. (1980) have not detected any permanent apertures in sinus-wall-lining cells in the rat, several other authors have reported the presence of gaps or pores along the parenchymatous sides of the sinus in murine (Moe 1963; Sakuma et al. 1981; Ushiki et al. 1995; Crivellato and Mallardi 1998), rabbit (Compton and Raviola 1985), and human (Forkert et al. 1977) lymph nodes. Through these gaps, processes of FRCs (Crivellato and Mallardi 1998) or processes of macrophages/histiocytes have been described to protrude (Sakuma et al. 1981; Ushiki et al. 1995). Such macrophage processes may capture material from the lymph and deliver it to immune cells in the parenchyma (Phan et al. 2007; Junt et al. 2007; Martinez-Pomares and Gordon 2007). These functional implications will be described in more detail below (see “Physiological aspects of SEVCs”).
In general, lymph node sinus appear to be bordered, at the parenchymatous side, by a trilaminar wall composed of (1) lining endothelial cells (SEVCs), (2) a layer of extracellular material including a (partly incomplete) basal lamina, some collagenous fibrils, and fewer fibrous structures suggestive of elastic fibers (e.g., Forkert et al. 1977), and (3) an incomplete layer of flattened FRCs with large gaps, forming the outer demarcation of the parenchyma. These FRCs are part of the general dendritic reticular meshwork of the lymph node. A subpopulation of FRCs, designated cytokeratin-positive interstitial reticulum cells (CIRCs; Franke and Moll 1987; Gould et al. 1995), contains IFs formed by keratins K8 and K18, and CIRCs may be included among the perisinusal FRCs.
Desmoplakin and other cytoplasmic plaque proteins of the complexus adhaerentes
The intense reaction of the SEVCs with desmoplakin antibodies is remarkably selective, as the reactions for all other desmosome-specific proteins such as plakophilins 1–3, desmogleins 1–4, and desmocollins 1–3 have been consistently negative (Schmelz and Franke 1993; Schmelz et al. 1994; Hämmerling 2004; Hämmerling et al. 2006). This is surprising as it defines a special type of cell-cell adhering junction sui generis, the complexus adhaerens, which is characterized by the occurrence of desmoplakin as an abundant component but out of context with the other desmosome-specific proteins. This is in contrast to the desmosomes of the near-by FDCs, which contain (in addition to desmoplakin and plakoglobin) plakophilin-2, desmoglein-2, and desmocollin-2, i.e. a complete desmosomal marker ensemble.
In the SEVCs of the subcapsular, intermediate, and medullary sinus, one notes, in addition to the desmoplakin reaction, general and intense immunostaining for a series of non-desmosomal junctional plaque proteins, such as α- and β-catenin that colocalize with (or are seen near to) desmoplakin in structures of various sizes, shapes, and patterns (“syndesmos” structures; Schmelz and Franke 1993). A typical example is presented in Fig. 2a,a’,b,c. Careful comparisons of the specific immunostaining profiles of the complexus adhaerens structures have shown colocalization of both desmoplakin and the catenins in some regions, whereas other sites appear positive only for one plaque protein or the other. These minor local differences, however, may simply reflect small local differences of antigen accessibility. A similarly intense reaction for desmoplakin, although always in small punctate structures, is regularly seen in the FDCs of the follicles (F in Fig. 2), which contain numerous small but complete desmosomes (Franke and Moll 1987; Nuber et al. 1995; Mertens et al. 1999) and similarly small distinct puncta adhaerentia junctions (see, e.g., Müller et al. 2000; Hämmerling 2004; Hämmerling et al. 2006). By contrast, the cells of the extrafollicular fibroblastic reticulum, FRCs, are negative for all specific desmosomal components and positive only for molecular markers of puncta adhaerentia, such as the catenins (in Fig. 2a, this is shown for β-catenin).
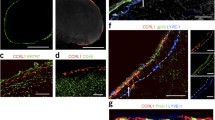
Double-label laser scanning immunofluorescence micrographs of a cryostat section through a human lymph node, showing the localization of β-catenin (a) and desmoplakin (a’) in the SEVCs of the sinus (S; left bracket in a’) and in dendritic reticulum cells (FDCs) of the follicle (F; right bracket in a’ denoting the germinal center), both of which are positive for both proteins, whereas blood vessels (two are denoted by arrowheads) are only β-catenin-positive. Note, in particular, the frequency of desmoplakin-positive SEVC protrusions extending into the lumen of the sinus, often ensheathing collagen fibril bundles (b-d details of desmoplakin immunostaining in such cell projections). The boxed cross-sectioned cell in a’ is shown at higher magnification in d and represents a prototypic example of a virgultar meshwork cell with processes that span the entire sinus. Middle bracket in a’, follicular mantle zone. Bars 50 µm (a, a’, b), 25 µm (d), 10 µm (c)
This restriction of desmoplakin to the SEVCs and its absence from the surrounding FRCs of the parenchymatous reticulum and from the endothelium of blood vessels is directly seen in the two-color immunofluorescence micrograph of Fig. 3a, which presents a double-label comparison with β-catenin. Here again, the SEVCs show regions of colocalization (yellow-orange merged color), whereas other sites are positive for only one of the two proteins. A similar situation is presented in Fig. 3b, in which the desmoplakin reaction of the SEVC junctions is directly compared with the immunoreaction of protein ZO-1, a characteristic marker for the cytoplasmic plaques of both adhering and tight junctions (see also “Tight junction proteins in complexus adhaerentes ”). Moreover, p120 catenin, another plaque protein of the armadillo family (for reviews, see Anastasiadis and Reynolds 2000; Hatzfeld 2005; McCrea and Park 2007), has also been identified in complexus adhaerens junctions (Fig. 4).
Double-label immunofluorescence laser-scanning microscopy (LSM) of desmoplakin (a, b red) compared with the adherens junction protein β-catenin (a, green) or the adherens and tight junction-associated protein ZO-1 (b, green). Desmoplakin and β-catenin colocalize in SEVCs of the sinus (S) at many sites (yellow-orange merge color) but are not fully coincident (a). The double-label staining of desmoplakin and protein ZO-1 in the sinus (S) also shows some sites of local colocalization (b). Blood vessel (V) walls are positive only for β-catenin (a) or protein ZO-1 (b). Bars 50 µm
An even broader distribution has been seen with antibodies against plakoglobin. This armadillo family protein, which generally can be found in both desmosomes and adherens junctions of epithelial and endothelial cells (Cowin et al. 1986; Franke et al. 1987, 1988, 1989a), also occurs in both the desmosomes and the puncta adhaerentia of the follicular dendritic reticulum (FDCs), in the puncta of the interstitial extrafollicular reticulum cells (FRCs), in the vascular endothelial zonulae adhaerentes, and in the complexus adhaerentes of the lymph node sinus (see, e.g., Hämmerling 2004; Hämmerling et al. 2006).
Reactions for the plakophilins, another subfamily of junctional plaque proteins of the armadillo type characteristically occurring in desmosomes, are markedly different (for reviews, see Heid et al. 1994; Schmidt and Jäger 2005; Hatzfeld 2007). In lymph nodes, only the desmosomes of the FDCs react positively for one of the plakophilins, and this has been determined to be exclusively plakophilin-2 (Mertens et al. 1999; Hämmerling 2004; Hämmerling et al. 2006).
The occurrence of desmoplakin in the absence of the other desmosome-specific constituents at endothelial cell junctions is not restricted to SEVCs of lymph nodes. Junctions with desmoplakin as a major component have also been identified in various other lymphatic endothelia, including small lymph vessels of the dermis and lingual mucosa (Schmelz et al. 1994; Ebata et al. 2001a, b; Hämmerling 2004; Hämmerling et al. 2006). Moreover, desmoplakin has also been identified in endothelial cells of blood vessels, notably in cell cultures, and appears to be a protein of general importance for vascular development (Valiron et al. 1996; Kowalczyk et al. 1998; Gallicano et al. 2001; Zhou et al. 2004; for reviews, see also Sleeman et al. 2001; Stacker et al. 2002; Ferreri and Vincent 2008).
Cadherins in complexus adhaerentes and other endothelial junctions of lymph nodes
As the SEVCs do not contain any of the typical desmosomal cadherins, i.e., desmogleins and desmocollins, the question of the transmembrane glycoproteins involved in the formation and maintenance of the complexus adhaerentes and, in particular, their cytoplasmic plaques is of central molecular and organizational importance. Again surprisingly, in our hands, the complexus adhaerens structures and the entire SEVCs have been negative for N-cadherin (Fig. 5), which, in the same sections, is constantly and intensely seen in the punctate adhering junctions of both kinds of reticulum cells, viz., the FDCs and extrafollicular FRCs, and in the vascular endothelial cell junctions (Hämmerling 2004; Hämmerling et al. 2006; for partly controversial views of the topology and functions of N-cadherin in adhering junctions of blood vessel endothelia, see, e.g., Salomon et al. 1992; Lampugnani et al. 1995; Dejana 1996, 2004; Lampugnani and Dejana 1997; Navarro et al. 1998; Luo and Radice 2005; for a claim of the coexistence of both N- and E-cadherin in cultured rat microlymphatic cells, see Hayes et al. 2003).
Double-label LSM of a cryosection through a human lymph node, showing colocalization of the adherens junction protein N-cadherin (red) and desmoplakin (green) in the follicular (F) dendritic reticulum. Note that, at the light-microscopic level, the yellow merge color represents the close apposition of the small puncta adhaerentia and desmosomes in the FDCs. The desmoplakin-positive SEVC system of the sinus (S) does not reveal significant reactions of N-cadherin, whereas the surrounding interstitial reticulum cells (mostly FRCs) are positive for N-cadherin, but not for desmoplakin. Bar 50 µm
In stark contrast, there is an intense reaction of VE-cadherin (cadherin 5) not only in the endothelia of blood vessels of lymph nodes, but also in all SEVCs, in the latter often in apparent colocalization with desmoplakin (Fig. 6a, b; for an indirect comparison with α-catenin, see Fig. 6c). We are therefore tempted to hypothesize that, in the complexus adhaerentes of SEVCs (and of lymphatic vessel endothelia), desmoplakin is involved in the anchorage, the homophilic association and lateral clustering, and the homotypic Ca2+-dependent cell-cell interactions of VE-cadherin (for some corresponding observations, see also Vittet et al. 1997; Ahrens et al. 2003), be it directly or through complexes with α-catenin or any of the armadillo proteins present (see, e.g., Breviario et al. 1995; Caveda et al. 1996, Carmeliet et al. 1999; and references cited therein).
Double-label immunofluorescence LSM of cross sections through frozen tissue of human lymph nodes, showing the specific mutually exclusive localization of VE-cadherin (a, red) in the endothelium of small blood vessels (V) and the punctate staining for desmoplakin (a’, green) in the dendritic reticulum cells (FDCs) of the follicle (F). Colocalization of desmoplakin and VE-cadherin, however, is seen in the complexus adhaerentes of the SEVCs of the sinus (S), as can be seen with special clarity (yellow) in the merged image (a’’; for a merged image at higher magnification, see b). A corresponding merged image showing colocalization of desmoplakin (red) and α-catenin (green) is presented in c. Bars 50 µm
Again, the reaction pattern of cadherin-11, which in other cells often occurs in coexistence with N-cadherin (e.g. Hinz et al. 2004; Wuchter et al. 2007; and references cited therein), including various cell types in lymph nodes (Hämmerling 2004), has been unexpected, as it is selectively negative on SEVCs (Fig. 7). The significance of this negative reaction has been controlled by the highly intense reactions of cadherin-11 in the trabecular fibroblastoidal cells (T in Fig. 7) and in the various interstitial cells of mesenchymal character located between the follicles and the sinus, including the FRCs (Fig. 7). It has also not escaped our attention that the trabecular cadherin-11 immunostaining correlates with an intense and specific reaction for certain collagen types (data not shown). Cadherin-11 is generally of widespread occurrence in diverse mesenchymal cell types, but neither in gene abrogation experiments nor in analyses of specific genetic mutations have definitive functions of this glycoprotein so far been elucidated to a satisfactory degree (e.g., Simonneau et al. 1995; MacCalman et al. 1996; Shibata et al. 1996; Simonneau and Thiery 1998; Horikawa et al. 1999; Hinz et al. 2004; Wuchter et al. 2007), except for some special roles in osteogenesis, chondrogenesis, and tendon development (e.g., Kawaguchi et al. 2001a, b; Luo et al. 2005; Richardson et al. 2007).
Double-label immunostaining LSM of cadherin-11 (red) in the fibroblastoidal cells forming the connective tissue of a trabecula (T), whereas the SEVC system (S, intermediate sinus) is distinctly positive for desmoplakin only (green). Note also positive immunoreaction of the cadherin-11 antibodies in the interstitial cells of the reticulum (mostly FRCs) that surrounds the sinus. Bar 50 µm
So far, we have been unable to localize protocadherin-12, i.e. VE-cadherin-2 (Telo’ et al. 1998; Rampon et al. 2005; see therein for further references), in any lymph node cell type. Another endothelial cadherin, cadherin-13 (previously also called V-cadherin; Haselton and Heimark 1997), has been reported in a subset of SEVCs of both human and bovine lymph nodes, but the significance and specificity of this reaction has not yet been ascertained (Schmelz and Franke 1993; Schmelz et al. 1994).
Therefore, we conclude that the complexus adhaerentes of SEVCs appear to be the only type of endothelial cell junctions based exclusively on VE-cadherin, i.e., without N-cadherin. Consequently, we consider it unlikely that, in the SEVC part of the vascular endothelial system, N-cadherin plays a master role in controlling VE-cadherin synthesis and functions, as has been proposed for other parts of the endothelial system by Luo and Radice (2005).
Proteins of the afadin ensemble
Using antibodies against proteins of this group, which include plaque proteins such as afadin and transmembrane components such as nectin (for a review, see Takai and Nakanishi 2003), we have obtained an intense reaction in SEVCs only for afadin, partly also in colocalization with desmoplakin (Fig. 8). Reactions for other proteins of this group, however, have so far been negative or their significance has not yet been definitively determined (Hämmerling 2004).
Double-label LSM comparing the immunolocalization of the plaque protein L-afadin (a, red) with that of desmoplakin (a’, green), which colocalize in the SEVCs of the two sinus (S) of a human lymph node. Note the distinct immunostaining of the small blood vessels (V) by L-afadin (in a and in the merged picture a’’). Bar 50 µm
Tight junction proteins in complexus adhaerentes
Certainly one of the greatest surprises in our analyses of the molecular composition of the complexus adhaerentes has been the discovery of the general occurrence of claudin-5 (Fig. 9a–d; see also Hämmerling et al. 2006), the hallmark protein of the endothelial tight junction. This tetraspan transmembrane protein is also present in cell-cell junctions of endothelia of blood vessels but is totally absent from all other lymph node structures, including the FDCs and the FRCs (Fig. 9a,a’). In the SEVCs, we have noted a wide-reaching but not complete overlap of claudin-5 immunofluorescence with that of desmoplakin, which defines the complexus adhaerens structures (Fig. 9a,a’,b,b’). Generally, the reaction for claudin-5 appears more extended and linear than that of desmoplakin (Fig. 9c,d; cf. Hämmerling 2004). Meanwhile, claudin-5 has also been identified in endothelia of other parts of the lymphatic system (Baluk et al. 2007; Pfeiffer et al. 2008). Occasionally a weak reaction of claudin-1 in SEVCs has also been seen with a rabbit antiserum but the significance of this observation has not yet been ascertained by the use of other reagents (Hämmerling 2004). In mouse SEVCs, in which claudin-5 has recently been confirmed, no claudin-1, claudin-3, or claudin-12 has been detected (Pfeiffer et al. 2008). Finally, we are aware that the discussion as to the occurrence of the transmembrane protein occludin in SEVC structures is also still controversial (see Table 1; see also Baluk et al. 2007; Pfeiffer et al. 2008; and references cited therein).
a, a’, b, b’ Double-label immunolocalization LSM on cryostat sections through human lymph nodes, showing that the tight junction protein claudin-5 (a, b) and desmoplakin (a’, b’) colocalize in SEVCs of the sinus (S), whereas the follicle (F) shows positive staining for desmoplakin only, which is specific for the desmosomes of the FDCs. Blood vessels (V) are positive for claudin-5, which is known as a tight junction protein characteristic of vascular endothelia. c, d Immunohistochemical demonstration of claudin-5 in a paraffin-embedded human pelvic lymph node (c), compared with desmoplakin staining (d), showing an intermediate sinus (c, V capillary blood vessel in a trabecula) and a medullary sinus (d) with a degree of sinus histiocytosis and hyperplasia of SEVCs. Note the predominantly linear claudin-5 staining of SEVCs (c) as opposed to the predominantly punctate desmoplakin staining (d). Bars 50 µm (a, a’, b, b’), 100 µm (c, d)
Further tight-junction-associated proteins identified in association with the complexus adhaerentes of SEVCs include members of the junctional adhesion molecule (JAM) family, transmembrane molecules of the immunoglobulin superfamily. In endothelia of blood and lymphatic vessels, two or all three archetypal JAM proteins (JAM-1 to JAM-3/JAM-A to JAM-C; for nomenclature, see, e.g., Mandell and Parkos 2005) have been demonstrated (Aurrand-Lions et al. 2001; Ueki et al. 2008; for details, see Pfeiffer et al. 2008). In SEVCs of human and bovine lymph nodes, JAM-1 (i.e., JAM-A) has been consistently detected in our laboratory (Hämmerling et al. 2006). In mouse lymph nodes, both JAM-1 (JAM-A) and JAM-2 (JAM-C) have been reported in cells of the subcapsular sinus (Aurrand-Lions et al. 2001), which most likely represent subcapsular SEVCs. Recently, the presence of JAM-A and JAM-C in subcapsular and medullary sinus-lining cells has been confirmed by double-label immunostaining experiments, whereas JAM-B has been reported to be absent (Pfeiffer et al. 2008). Since, among the JAMs, only JAM-2 (JAM-C) seems to be restricted to endothelial cells (Aurrand-Lions et al. 2001), it may serve as an additional endothelial marker, at least in the mouse. In this species, another endothelial tight junction molecule related to the JAMs, viz., endothelial-cell-selective adhesion molecule (ESAM)-1, has also recently been identified in SEVCs (Pfeiffer et al. 2008) and proposed to be involved in cell migrations through the sinus-lining cell layer.
Finally, SEVCs produce the plaque protein ZO-1 (Fig. 3; cf. Hämmerling et al. 2006), which also occurs as a general component in the lymphatic endothelium (Hämmerling et al. 2006) and in tight and adhering junctions of blood vascular endothelial cells (Dejana 2004). Thus, SEVCs contain a set of tight junction proteins that overlaps with that found generally in lymphatic and blood vessel endothelia, with the possible exception of occludin. We should, however, mention, as a note of caution, that the exact spatial relationship of the tight junction elements to the total complexus adhaerens structures is not yet clear (Hämmerling et al. 2006), and hence more detailed immunoelectron-microscopical and biochemical investigations are required. Specifically, at present, we cannot rule out the possibility that these tight-junction-typical transmembrane proteins are concentrated in a narrow curvilinear intramembranous “ridge” confinement of the complexus. Moreover, the importance of the tight junction proteins for the barrier versus permeability functions of the SEVCs remains to be elucidated.
Table 1 summarizes the unique pattern of major molecular components constituting complexus adhaerentes in comparison with the molecular composition of adhering junctions of blood vessel endothelia. Complexus adhaerentes are therefore specifically characterized by the presence of desmoplakin together with the absence of N-cadherin and protein p0071.
Electron microscopy of cell-cell connections in the SEVC system
In the lymph nodes of the mammalian species examined so far, the sizes and architectural organization patterns of the complexus adhaerens structures of SEVCs differ markedly. Frequently, the relatively large junctional arrays characterized by their typical electron-dense cytoplasmic plaques show local interruptions of variable sizes, shapes, and plaque coating. Figure 10 illustrates this in a series of eight consecutive ultrathin sections (four of them shown), revealing small plaque-free regions (some are denoted by arrows). Detailed morphological and morphometrical studies, including image reconstructions, have previously been published (e.g., Schmelz and Franke 1993; Franke et al. 1994; Schmelz et al. 1994). Notably, bundles of typical actin microfilaments or of IFs of the vimentin type (Franke et al. 1979) often lie close to these plaques, sometimes suggestive of “loose” lateral association rather than of terminal anchorage.
Electron micrographs presenting a series (a-d) of consecutive ultrathin sections through a region of a bovine lymph node in which SEVCs are attached to each other (numbers lower right give the specific section number in the series). The two cells shown are connected by a large complexus adhaerens, characterized by a dense cytoplasmic plaque and an irregular pattern of plaque interruptions (see arrows in b, c). Bar 0.5 µm
Figure 11 presents a series of images showing the often winding and deeply interdigitating regions of these complex adhering junctions, frequently with small or larger interruptions of the plaque-coated plasma membranes (e.g., Fig. 11a,e–g,h,h’; note that similar junctional arrays, including those with multiple deep interdigitations, also occur in other vascular endothelia; for a review, see Franke et al. 1988). In some regions, these plaques show remarkable local fluctuations in size and electron density, often suggestive of a higher order organization of more or less closely-packed plaque elements (Fig. 11b–d). In other regions, such cell-cell junction complexes appear as individual domains with widely variable diameters (e.g., Fig. 11e). In some plasma membrane convolutions, the local packing density of the associated bundles of microfilaments and vimentin IFs is high (see Fig. 11c,e,f).
Electron micrographs of ultrathin sections through various syndesmos structures of complexus adhaerentes, showing adhering junctions of human and bovine SEVCs (a-h’) and the localization of desmoplakin (i-k), in conventional (a-h’) and immuno- (i-k) electron microscopy (C collagen fibers). a-h’ The often convoluted complexus adhaerentes appear in some places as large continuous structures (b), whereas in others, small substructures can be recognized (c, arrows; see also d). They sometimes appear as a complex of larger and smaller puncta adhaerentia-like structures (arrowheads in e; see also the substructures discernible in f). Note the beginning and the end of a cell-cell-contact (thin arrows in g) that seems to be composed of variously sized subdomains (arrowheads). Two adjacent ultrathin sections of a series (h, h’) demonstrate local differences in complexus adhaerentes (arrows). The basal lamina is denoted by thick arrows (bottom) in e–g. i-k Immunogold-silver enhancement reaction, showing the plaque localization of desmoplakin in the complexus adhaerens structures. Bars 0.5 µm (a, c–g, i), 0.2 µm (b, h, h’,k), 1 µm (j)
Irrespective of the specific size and morphology of these complex junctions, immunoelectron microscopy has generally shown positive reactions for desmoplakin (e.g., Fig. 11i–k), β-catenin, and any of the other complexus adhaerens components examined (data not shown).
As the architecture of a specific sinus appears to be influenced by the position, thickness, and length of the trabecular structures and corselet-like “stays” of paracrystalline collagen fiber bundles extending into the luminal spaces, we have taken special care to elucidate the intimate relationship between these semiplastic collagen and elastin fibril bundles and the surrounding SEVC processes (for earlier electron microscopy, see Moe 1963; Forkert et al. 1977; Compton and Raviola 1985; Yoshida and Takaya 1992; Wacker 1994; Crivellato and Mallardi 1998). Our major findings are summarized in Fig. 12, showing a tomographic stack of electron micrographs of cross sections through such a paracrystalline bundle comprising, in this case, ten collagen fibers, lying closely together with near equidistant spacing, in six sections of a total series of ten. The entire length of this collagen fiber bundle, with some surrounding loose arrays of elastin fibrils, is ensheathed by a manchette of flattened virgultar cell processes that are tightly connected by a complex of small adhering junctions (Fig. 12a–f, arrowheads). The plaque-bearing portions of these puncta adhaerentia-like junctions mostly appear to be closely attached, whereas in other small regions plaque-free areas can also be discerned (Fig. 12c–f). From such electron micrographs and the desmoplakin immunostaining, we conclude that the collagen-fiber-based “stay” structures of lymph node sinus are, over large areas, tightly enveloped by SEVC processes and “stitched” together by complexus adhaerentes. In most of the cases examined, the endothelial processes forming extended manchettes resemble the “fibripositors” that have been described with respect to the relationship of fibroblast cell protrusions and collagen fibril bundles in tendon formations (Canty et al. 2004, 2006; Richardson et al. 2007; see also Birk and Trelstad 1986).
a-f Electron micrographs showing six images from a series of ten consecutive cross sections through one of the typical “stays” of collagen fiber bundles (arrow) tightly enwrapped by cytoplasmic projections of SEVCs. The paracrystalline bundle of ten collagen fibers shown here is intimately surrounded by projections from two cells forming an extended array of complexus adhaerentes (distinct substructures are denoted by arrowheads). Note that the collagen-fiber-containing canal also harbors thinner filamentous structures. Bar 0.3 µm
For the sake of clarity, we should emphasize that these collagen fibril bundle arrays ensheathened by manchette-like cell processes should not be confused with the collagen-based reticular fibers that are only surrounded by elastin fibers, with or without several typical basement-membrane-specific components but not with cell processes (see, e.g., Kramer et al. 1988; Hayakawa et al. 1990).
In summary, the complexus adhaerens is a unique, extended, complex, variously sized and shaped junction structure covering large parts of adjacent cell borders. This is illustrated in Fig. 13, which presents a “typical” tomographic reconstruction model (cf. Schmelz and Franke 1993) showing a small section of a syndesmos structure connecting two SEVCs in the sinus of a bovine lymph node.
Computer-aided model reconstructed from electron micrographs of a tomographic series of sections through an extended syndesmos region between two virgultar SEVCs in the sinus of a lymph node. Plasma membranes of the cells are shown in blue; one of the cytoplasmic plaques of the junction is shown in purple. Note the fenestrated structure and the extent of the cytoplasmic plaque (from Schmelz and Franke 1993)
Complexus-adhaerens-related junctions in other parts of the lymphatic vessel system
The typical variously sized and shaped complexus adhaerens (see representation in Fig. 13) is a characteristic structure of SEVCs of the lymph node sinus. By using, however, desmoplakin as an immunohistochemical marker protein in a search for complexus-adhaerens-related endothelial junctions in other tissues, such structures have also been identified in several parts of the lymphatic vessel system in the various mammalian species studied, in particular in the spleen and in small lymphatic vessels of the dermis, of the submucosae of various organs, and of muscle tissue (Schmelz et al. 1994; see also Ebata et al. 2001a, b). These observations have recently been extended to further junctional molecules and related to certain lymphatic functions and pathogenic processes (cf. Baluk et al. 2007; Pfeiffer et al. 2008). In view of the general importance of the lymphatic system in homeostasis and in pathogenic processes, notably in cancer metastasis (for recent reviews, see Stacker et al. 2002; Al-Rawi et al. 2005; Alitalo et al. 2005), the crucial contributions of the lymphatic endothelial junctions in general and in the lymph node sinus in particular should now be elucidated by using specific molecular markers as a morphology-independent criterium (cf. Table 1).
Endothelial cell type markers in SEVCs
As the endothelial nature of the lymph node SEVCs has been repeatedly doubted or even denied (for reviews, see Wacker 1994; Gretz et al. 1997; Willard-Mack 2006), we have characterized these cells by immunohistochemistry with specific molecular markers for endothelial cells in various mammalian species (cf. Schmelz and Franke 1993; Hämmerling 2004; for lymphatic endothelial markers, see also Podgrabinska et al. 2002). Table 2 summarizes our findings and those of other authors. The occurrence of endothelium-specific cell junction proteins such as VE-cadherin, claudin-5, and JAM-2/JAM-C (mouse) in SEVCs has previously been mentioned (see Table 1). With respect to classical endothelial cell markers (Table 2), a typical result showing double-label immunofluorescence microscopy of the factor-VIII-related antigen (von Willebrand’s factor) and desmoplakin is presented in Fig. 14a,b. Clearly, the desmoplakin-positive SEVCs are also positive for the factor-VIII-related antigen (Schmelz and Franke 1993). The significance of this reaction is underscored by the absence of the factor-VIII-related antigen in the desmosome-positive FDCs. Vice versa, however, the blood vessel endothelial cells in the lymph node parenchyma are positive for factor-VIII-related antigen but negative for desmoplakin (Fig. 14a,b). In contrast to the junctional pattern of desmoplakin, immunostaining for factor-VIII-related antigen typically exhibits a finely granular cytoplasmic appearance (Fig. 14a,c; Schmelz and Franke 1993; for electron microscopy of factor-VIII protein in endothelial cells, see, e.g., Simionescu and Simionescu 1988). Remarkably, whereas in blood vessel endothelium, this staining is mostly strong and uniform, in SEVCs, it often appears weaker and more variable (Fig. 14b; see also Schmelz and Franke 1993; Krenács and Rosendaal 1995). In addition to the distinct granular staining, some SEVCs, both in bovine lymph nodes (Fig. 14a) and in human lymph nodes from diverse sites, including pelvis (Fig. 14b,c), abdomen and axilla (not shown), exhibit factor-VIII-related antigen as conspicuous, densely stained, paranuclear aggregates, an aspect that remains to be clarified by immunoelectron microscopy. To the best of our knowledge, Weibel-Palade bodies, which harbor factor-VIII-related antigen in combination with other factors important for blood coagulation in blood vessel endothelial cells (Simionescu and Simionescu 1988), have not yet been mentioned in ultrastructural studies of SEVCs.
Localization of the endothelial marker, factor-VIII-related antigen (a), in comparison with that of desmoplakin (a’) in double-label LSM of an acetone-fixed cryosection of a bovine lymph node and in immunohistochemical staining of paraffin sections of human lymph nodes (b, c). C capsule. Both factor VIII protein and desmoplakin occur in SEVCs (S sinus). In addition, exclusive staining of blood vessels (V) is seen for factor VIII protein, whereas desmoplakin also occurs in the small desmosomes of the follicular (F) dendritic reticulum (lower right in a’). Note that both factor-VIII-related protein and desmoplakin can occur in the same SEVCs, although in obviously different cell substructures (e.g., plaque-like immunoreactivity indicated by arrows in a). Immunostaining of formalin-fixed and paraffin-embedded iliac lymph nodes (b, c) for factor-VIII-related antigen is strong in blood vessel endothelium (V) but often weaker and more heterogeneous in SEVCs (bracket in b subcapsular sinus), with some SEVCs apparently being negative (cf. Schmelz and Franke 1993), whereas variable numbers of SEVCs exhibit a conspicuous plaque-like immunoreactivity (b, c; some aggregates are denoted by arrows). Bars 50 µm (a, a’), 100 µm (b, c)
A further general endothelial cell marker, which also has been noted in certain blood and immune cells including macrophages, is the transmembranous glycoprotein CD31, also known as platelet endothelial cell adhesion molecule-1 (PECAM-1). In endothelial cells, this cell adhesion molecule of the immunoglobulin (Ig) superfamily, which sometimes appears to be enriched at cell-cell junctions, seems to serve multiple functions including transendothelial migration of leukocytes, angiogenesis, and apoptosis (Jackson 2003). It is also found in endothelial cells of lymphatic vessels, although the staining intensity is weaker in comparison with the blood vessel endothelium (Ebata et al. 2001b). Antigen CD31 has also been reported to occur in SEVCs of human and mouse lymph nodes (human: Ruco et al. 1992; Krenács and Rosendaal 1995; mouse: Aurrand-Lions et al. 2001; Singer et al. 2005), although Wacker et al. (1997) have not detected it in human sinus-lining cells on cryostat sections. Previously, we have reported strong staining of SEVCs of human lymph node sinus with monoclonal antibody EN4 (Schmelz and Franke 1993), which has been shown to recognize CD31 (Burgio et al. 1994). When we compared sections of human lymph nodes stained for desmoplakin with CD31 immunostaining in subsequent sections, we found CD31 consistently in all SEVCs, with mostly linear staining of the cell surfaces (Fig. 15a; see also below). In addition to desmoplakin and claudin-5, CD31 can thus be used as a sensitive marker of the SEVC system in lymph nodes. The functional roles of CD31 in SEVCs might include the adhesion and transmigration of leukocytes.
Immunostaining of SEVCs by endothelial markers as demonstrated in a subcapsular sinus of human formalin-fixed and paraffin-embedded abdominal (a), axillary (b), and cervical (c) lymph nodes (C capsule, S subcapsular sinus, V small blood vessels with their endothelial cells markedly positive for all three antigens). Antigen CD31 (a; clone JC70A) appears in an extended, predominantly linear, surface staining of both the sinus-lining and the virgultar SEVCs and their cell processes, whereas staining for CD34 (b; clone QBEND-10) is much more restricted to a minor subpopulation of SEVCs (other areas are completely negative; not shown). Thrombomodulin (CD141; c; clone 1009) reveals a heterogeneous pattern, with predominance of sinus-lining SEVCs (at the parenchyma face contiguous with some reticulum cells), whereas a few intrasinusal SEVCs are also positive. Bar 100 µm (in c)
CD34 is a sialomucin constitutively produced in most blood vascular endothelial cells and, as an L-selectin ligand, is involved in the binding of leukocytes. As to the lymphatic vascular system, CD34 appears to be absent from lymphatic endothelium in normal tissue, whereas it is prominent in endothelial cells of lymphatic vessels of tumors and of chronically inflamed tissues and thus may be regarded as an activation marker antigen of human lymphatic endothelial cells (Fiedler et al. 2006). In SEVCs of human lymph nodes, we detected CD34 only at low degree and usually only a small subset of these cells was positively immunostained (Fig. 15b). In some lymph nodes, the SEVCs were completely CD34 negative, and we did not observe any correlation between the CD34 status of SEVCs and the activation or regression state of the lymph nodes (Sievers 2009).
When analyzing thrombomodulin (CD141), a cofactor for the thrombin-mediated activation of protein C (Suzuki et al. 1987) and another marker for blood and lymphatic vessel endothelia, we observed a remarkable expression pattern. A variable subpopulation of SEVCs, with some preference of sinus-lining SEVCs, was intensely stained with anti-thrombomodulin antibodies, as were some adjacent perisinusal cells in the parenchyma probably representing FRCs (Fig. 15c).
Other endothelial components examined, including CD105 (also known as endoglin; Duff et al. 2003) and CD143 (also known as ACE, i.e., angiotensin converting enzyme; Danilov et al. 1991), distinctly reacted only with certain subsets of blood vessel endothelial cells. We did not detect these markers on SEVCs (not shown). Binding of the lectin Ulex europaeus agglutinin-I (UEA-I), a further marker of blood vessel endothelial cells, was negative in the sinus of the lymph nodes studied, and here, in contrast to vascular and lymphatic endothelia, SEVCs apparently did not bind UEA-I (not shown; see also Wacker 1994). On the other hand, SEVCs have been reported to be positive for BMA 120, another marker of blood vascular endothelial cells (Kaiserling et al. 1998). Thus, one can conclude that SEVCs present several fundamental markers of blood vascular endothelial cells, whereas others appear only as minor elements or are absent.
Several specific markers of lymphatic endothelial cells have recently become available that are of particular interest with respect to SEVCs as a part of the lymphatic vascular system. An especially striking and specific example is provided in Fig. 16a, showing a double-label immunofluorescence microscopic comparison of desmoplakin with the lymphatic marker protein LYVE-1 (lymphatic vessel endothelial hyaluronan receptor-1; cf. Banerji et al. 1999; Podgrabinska et al. 2002). Whereas the SEVCs in the sinus are positive for both LYVE-1 (see also Jackson et al. 2001; Wróbel et al. 2005; Hämmerling et al. 2006; for the mouse, see Phan et al. 2007) and desmoplakin, the FDCs are exclusively positive for desmoplakin (Fig. 16a). In some cases of paraffin-embedded human lymph nodes, LYVE-1 staining of SEVCs has been markedly heterogeneous, in contrast to homogeneous staining of lymphatic vessel endothelium (not shown). Further support for the true lymphatic endothelial character of SEVCs is provided by our finding (Fig. 16b) that these cells stain positively for vascular endothelial growth factor receptor-3 (VEGFR-3, FLT4), a molecule involved in the development of lymphatic endothelia (Kaipainen et al. 1995). Another widely used lymphatic endothelial marker is podoplanin, a small extensively O-glycosylated membrane glycoprotein essential for the development of lymphatic vessels and established as a marker to discriminate between blood and lymphatic endothelial cells (Breiteneder-Geleff et al. 1999). Here, we detected podoplanin by using monoclonal antibody D2–40, which has recently been reported to recognize human podoplanin specifically (Schacht et al. 2005), and obtained variable results in SEVCs. Whereas endothelia of small perinodal lymphatic vessels were consistently positive (Fig. 16c), abundant podoplanin staining of SEVCs (Fig. 16c) was the exception, with usually only occasional SEVCs being immunostained (cf. Fig. 16d).
a Double-label LSM of a human lymph node (cryostat section) for the lymphatic vascular endothelial protein (LYVE-1) (red) and desmoplakin (green), which colocalize (yellow) in the SEVCs of the sinus (S); the follicle (F) shows positive staining for desmoplakin only, reflecting the numerous small desmosomes of the FDCs. Note also the incomplete match of the two proteins at certain sites of the SEVC meshwork. b Positive immunostaining of SEVCs, after histochemistry, in a section through a paraffin-embedded human pelvic lymph node is also seen for VEGFR-3 (clone 9D9) in an intermediate sinus (T trabecula), whereas blood vessel (V) endothelium is essentially negative. c, d On similar sections (intraparotideal lymph node), podoplanin, as recognized by monoclonal antibody D2–40, shows highly variable staining of SEVCs. Exceptionally abundant staining is seen in c, whereas SEVCs in many other areas are negative or reveal only scarce podoplanin-positive cells, in this case, at the parenchymatous face of the sinus wall (arrows in d). Note the strong endothelial staining of a capsular (C capsule) lymph vessel (L in c). Bars 50 µm (a), 100 µm (b-d)
Thus, based on the presence of a distinct spectrum of general endothelial markers and several lymphatic endothelial markers (Tables 1, 2), SEVCs should be regarded as a special subtype of endothelial cells related to but not identical with lymphatic vessel endothelium.
With respect to the differences in molecular composition between the adhering junctions connecting endothelial cells of blood vessels, on the one hand, and the complexus adhaerentes of the SEVCs, on the other (cf. Table 1), the most remarkable is certainly the presence of desmoplakin in the adhering junctions of SEVCs (see Figs. 2, 3, 5, 6, 7, 8, 9, 14, 16, and below) and the absence of significant amounts of protein p0071 in these cells, as emphasized by results of their double-label immunoreaction (Fig. 17). In contrast, p0071 is readily detectable in the vascular endothelia of vessels of diverse calibers (Fig. 17, arrows; see also Hofmann et al. 2008). The presence of protein p0071, which is a catenin-type protein of the armadillo family and a constituent of various types of adhering junctions (Hatzfeld and Nachtsheim 1996; Hatzfeld 2005), including vascular endothelia (Calkins et al. 2003), now provides a surprising difference between blood vessel and lymphatic endothelia. We thus suggest the use of p0071 as a molecular marker for a subset of endothelia, but excluding the SEVC system.
Double-label LS microscopy of a cryostat section through a human lymph node (T trabecula) immunostained for LYVE-1 (green) and the adhering junction plaque protein p0071 (red), showing the presence of this junctional protein in the endothelia of blood vessels of various calibers (arrows), in contrast to its absence in the SEVCs of an intermediate sinus (green). Bar 50 µm
In addition to the various endothelial markers, several other biologically important antigens have been detected in SEVCs, including connexin 43 (Cx43), a component of gap junctions, which appears in a distinct dotted immunostaining pattern in these cells and in FDCs (cf. Krenács and Rosendaal 1995). SEVCs are also stained by Ki-M9, a monoclonal IgM antibody recognizing a unique membrane-bound protein of 70 kDa with a short half-life (Wacker et al. 1997; Middel et al. 2002). This antibody also more weakly stains FDCs, and on the basis of these and other data, Wacker et al. (1997) have postulated an ontogenic relationship between SEVCs and FDCs and a possible function of SEVCs as antigen-presenting and accessory cells in the primary humoral immune response (Wacker et al. 1997; Middel et al. 2002). Another feature that SEVCs share with FDCs is the binding of the lectin Chelidonium majus agglutinin (CMA), but other cell types in lymph nodes, including FRCs and blood vessel endothelial cells, also produce carbohydrate moieties that bind to CMA (Düllmann et al. 2002). Moreover, cells of the medullary sinus, probably SEVCs, have been described to be positive with a monoclonal antibody to langerin (CD207; Chikwava and Jaffe 2004), a type II lectin specific for antigen-capturing, -processing, and -delivering Langerhans cells. The same has been found for the lining cells of hepatic sinusoids. Both sinus-lining cell types lack, however, the Langerhans cell antigen CD1a. Moreover, S-100 protein, typically present in Langerhans cells and interdigitating dendritic cells (IDCs), is absent from SEVCs. Certain antigens characteristic of hematopoietic cells may also be present in SEVCs. Thus, CD45 (leukocyte common antigen), a transmembrane glycoprotein expressed on most nucleated cells of hematopoietic origin, may be found immunohistochemically on the surface of a subpopulation of SEVCs (not shown). This has previously also been been noted by Wacker (1994), who has interpreted this feature as suggesting a hematogenous origin of these cells. Immunostaining for the macrophage marker CD68 sometimes yields a finely granular cytoplasmic reaction in SEVCs (not shown), the significance of which remains to be proven.
Molecular complexes and functional protein interactions in the complexus adhaerens: hypotheses deduced from other adhering junctions
The complexus adhaerens represents a remarkable ensemble of junctional molecules that otherwise are known as constituents of different kinds of junctions (Table 1). In addition to VE-cadherin and typical adherens junction plaque proteins, the complexus comprises large amounts of desmoplakin, generally known as a hallmark protein of epithelial desmosomes and related cardiomyocytes junctions, as well as claudin-5 and protein JAM-A, i.e., typical tight junction components. So far, practically no data are available as to the molecular complexes present in the complexus adhaerens structures (chemical cross-linking and immunoprecipitation experiments on micro-dissected portions of lymph node tissue are currently underway in our laboratories). On the other hand, hypotheses related to the possible functions of the major complexus adhaerens molecules may be deduced from findings in other endothelia, including cell culture and gene abrogation experiments (for recent reviews on adherens and tight junctions, see Ebnet et al. 2003; Aijaz et al. 2006; Ebnet 2008; for tight junctions, see also Burns et al. 2000).
Thus, functional contributions of intact or truncated VE-cadherin, alone or in interaction with specific junctional plaque components, in the formation of adhering junction structures and in the control of endothelial organization, permeability, and transmigration phenomena have been repeatedly reported (e.g., Del Maschio et al. 1996; Navarro et al. 1995, 1998; Hordijk et al. 1999; Corada et al. 1999, 2001; Shaw et al. 2001). Remarkably, from “competition in situ” experiments by cell transfections with VE-cadherin cDNA constructs, Jaggi et al. (2002) have concluded that the affinity of VE-cadherin for other members of the adherens junction ensemble is so strong that it can displace N-cadherin from its original structural junction complexes. Moreover, VE-cadherin has also been shown to occur in complexes with non-cadherin molecules such as certain protein kinases, phosphatases, the vascular permeability factor or vascular endothelial growth factor (VEGF), and such complexes have been discussed with respect to functions in the regulation of cell permeability, cell transmigration, and other “fence and tissue barrier” phenomena, and in specific intra- and intercellular signalling pathways, in some cases apparently via influences on the phosphorylation patterns of certain junctional proteins (e.g., Allport et al. 1997; Haselton and Heimark 1997; Alexander et al. 1998; Kevil et al. 1998; Ratcliffe et al. 1999; Johnson-Léger et al. 2000; Ukropec et al. 2000; Wong et al. 2000; Ferber et al. 2002; Iyer et al. 2004; Lambeng et al. 2005).
Several molecules, including certain growth factors (e.g., VEGF), are known to induce endocytic internalization of VE-cadherin, also via a clathrin-dependent pathway (e.g., Xiao et al. 2003, 2005; Gavard and Gutkind 2006; for discussion, see also Yap et al. 2007), and obviously, the greatly enhanced cellular surface of the SEVCs would provide a gigantic endocytotic capacity.
On the other hand, an influence of certain cadherins and widespread junction plaque-associated cytoskeletal proteins such as actin, vinculin, and α-actinin on some tight junction components and functions has been reported from experiments in rat blood-brain microvessels (e.g., Schulze and Firth 1993; Lampugnani et al. 2002). In rigorous embryogenesis experiments on the role of VE-cadherin in vascular morphogenesis, the fundamental and manifold importance of this cadherin has been amply demonstrated (e.g., Vittet et al. 1997; Carmeliet et al. 1999; Gory-Fauré et al. 1999; for a recent review, see Ferreri and Vincent 2008; for endothelial stem cells, see the review of Kubo and Alitalo 2003). In general, however, it is of course questionable whether and to what extent results obtained with other kinds of endothelia, i.e., in most cases flat, tightly closed endothelial cell monolayers, are comparable with or relevant for those of the complexus adhaerentes, in particular in the three-dimensionally organized and branched virgultar system. Here, endothelial permeability and the control of cell transmigration seem less likely to be important functions.
Consequently, one might expect that relevant functional relationships of the SEVC system will be discovered only in situ, i.e., in lymphatic structures of whole animals (for example, by alterations of the complexus ensemble after induction by cell-type-targeted gene abrogation or interference, also including the introduction of externally added substances). Of course, a special problem is presented here by the abundant desmoplakin in the context of the other desmosome-specific molecules. Clearly, desmoplakin can efficiently bind to, and be positioned by, VE-cadherin, directly or via other junction molecules (e.g., Kowalczyk et al. 1998; see also Valiron et al. 1996). Desmoplakin is also known to be of general importance in the embryogenesis of the vasculature (e.g., Gallicano et al. 2001; Zhou et al. 2004). Moreover, in other kinds of junctions such as in the area composita of cardiomyocytes in the forming embryonic heart, desmoplakin is normally bound to (and topologically recruited by) plaque-bound plakophilin-2 (Grossmann et al. 2004; for the importance of desmoplakin in assemblies of adhering and other junctions, see also Gallicano et al. 2001; Goossens et al. 2007; Koeser et al. 2003; Oxford et al. 2007; Pieperhoff et al. 2008). Thus, the complexus adhaerens, in which we have not yet detected any plakophilin-2 (probably small amounts of this general and widespread protein occur in nucleoplasmic regulatory complexes; see, e.g., Mertens et al. 1996, 2001), presents especially challenging questions as to the binding partners of desmoplakin and its lymphatic vessel-specific roles.
Physiological aspects of SEVCs
During lymph flow through the lymph node sinus, there is extensive contact of lymph fluid and lymph-borne cells with the SEVC system, suggesting that this system must have significant functional importance. The special morphological and molecular features of the SEVC system should obviously influence the interactions of lymph components with the SEVC meshwork and the permeability of the sinus wall structures.
The three-dimensional virgultum formed by intrasinusal SEVCs is a unique histologic feature in the mammalian body. Since the fibrillar elements of the extracellular matrix material spanning the sinus are completely and tightly covered by SEVCs and their processes, interconnected by complexus adhaerentes, the reactive surface of sinus cells is highly expanded, allowing more intense contact of the lymph and its constituents with the SEVCs. Consequently, the much enhanced lymphatic endothelial cell surface in the SEVC system should also enhance the binding and enrichment of lymphocytes as mediated by cell-cell receptors such as CLEVER-1 (Irjala et al. 2003). We might also assume that the jungle of SEVC processes in the lymph node sinus reduces the flow speed of the percolating cells, particles, and molecules, thereby facilitating the recognition, monitoring, endocytosis, and further processing of the incoming components of the lymphatic fluid. Certainly, this also supports the filter function of lymph nodes exerted by the phagocytic activity of sinus macrophages, which filter particulate material out of the lymph (for a review on scavenger function uptake by endothelial cells, see Smedsrød 2004). In rat lymph nodes, Sainte-Marie and Peng (1986) have specifically shown that lymph-carried fluorescence-labeled antigen proteins (albumin, Igs) are retained in certain parts of the subcapsular sinus, where they are associated with either cells lining the inner sinus wall or intrasinusal SEVCs.
In these experiments, certain fluorescently labeled antigens have been seen later to enter the parenchyma, along reticular fibers (Sainte-Marie and Peng 1986), pointing to the second major role of the SEVC system, i.e., the permeability of the parenchyma-bordering sinus walls with their lining layer of SEVCs, which is of utmost importance for the immunologic functions of lymph nodes. Obviously, lymph-borne diffusible antigens and particles and antigen-presenting cells must be able to reach the parenchyma, and T-lymphocytes must be able to leave the parenchyma to reach the sinus lymph system. The existence of gaps or pores in the SEVC layer lining the parenchymatous sides of the sinus seems now established (for references, see above), and processes of FRCs and in particular macrophages have been described to protrude into these intercellular spaces (e.g., Sakuma et al. 1981; Ushiki et al. 1995); such cells may well migrate and pass through the sinus wall. Several recent elegant function-oriented studies in mice have indeed shown that sialoadhesin (CD169, MOMA-1)-containing macrophages form cell processes that protrude between SEVCs and that capture immune complexes or viral particles from the lymph and deliver such material to B-cells in the parenchyma (Phan et al. 2007; Junt et al. 2007; Martinez-Pomares and Gordon 2007). However, in spite of the presence of pores or gaps in the SEVC layer lining the parenchymatous walls of sinus, injection experiments in mice and rats have also indicated the existence of a functional barrier restricting the percolation of soluble lymph-borne molecules of >70 kDa from the subcapsular sinus into the lymph node cortex (Gretz et al. 2000). Recently, Pfeiffer et al. (2008) have demonstrated that lymph-borne fluorescently labeled, large dextran (high-molecular weight, 2000 kDa) is strictly retained in the subcapsular and medullary sinus and does not enter the parenchyma. However, small molecules and particles (<70 kDa, e.g., 10-kDa dextran) can apparently penetrate the sinus walls and gain access to the cortex (Gretz et al. 2000) and to the paracortex and medullary regions of the parenchyma (Pfeiffer et al. 2008). How can the presence of such structural gaps or pores in the sinus-lining SEVC layer be compatible with its barrier function to large molecules and to particles? What functional role do the SEVC junctions, notably the complexus adhaerentes with their tight-junction-type components, play in the control of the sinus wall permeability? These questions remain to be answered.
Of course, sinus walls also need to allow the passage of various types of cells between the lymph and the parenchyma, in both directions. For example, recirculating T-lymphocytes migrate from high endothelial venules into intermediate sinus, thereby crossing the sinus wall. Specifically, lymphocyte transmigration across the sinus-lining cell layer appears to be an active process during which these cells assume a distorted hourglass morphology (for references, see Gretz et al. 1997; Johnson-Léger et al. 2000). Recently, sphingosine 1-phosphate (S1P) receptors located on endothelial cells (SEVCs) lining the medullary sinus have been shown to control the passage of T-lymphocytes from the medullary cords through pores in the sinus wall into the sinus lumen, with transient deactivation of the receptor complexes opening these gates (Wei et al. 2005). Singer et al. (2005) reported that treatment of mice with the immunosuppressant FTY720, a S1P receptor agonist, resulted in an enhanced production of the junctional molecules CD31, β-catenin, and protein ZO-1, all colocalized in intercellular junctions of lymphatic endothelial cells (SEVCs) of subcapsular sinus, suggestive of a special regulatory system in the subcapsular sinus-lining cell barrier (Singer et al. 2005). Apparently, according to the immunofluorescence data shown, this process was also accompanied by hyperplasia of the SEVCs.
Furthermore, dendritic cells (DCs) floating in the lymph are well-known to be able to penetrate the sinus wall actively, including the lining SEVC layer, and to find their way into the parenchyma (for references, see Willard-Mack 2006). SEVCs may also have adhesive properties by which macrophages can be trapped in the meshes of the luminal network of the intrasinusal SEVC system and apparently stick to the surface of these cells (Compton and Raviola 1985); obvious candidates for such adhesive functions include CD31 and LYVE-1.
Fibroblastoid and sinus endothelial/virgultar cells of lymph nodes: pathological changes and autochthonous tumor formations
Non-tumorous changes
Whereas lymph nodes play a major role in human pathology, relatively little is known about the pathology of SEVCs. Non-neoplastic changes involving the sinus include sinus catarrh and sinus histiocytosis. Sinus catarrh is a reactive condition that might be attributable to bacterial infections and is characterized by dilated sinus spaces, containing lymph fluid together with lymphocytes and other leukocytes (Fig. 18a). In such cases, hyperplasia of intrasinusal SEVCs, here outlined by the SEVC marker CD31 (Fig. 18b), is noted, as has also previously been described by Wacker (1994) using Ki-M9 as a marker.
Altered distribution patterns of SEVCs in reactive pathological changes of human lymph nodes. Sinus catarrh (a, b mesenterial lymph node) is characterized by distended sinus with some floating cells and also presents a SEVC hyperplasia (a H&E staining; b CD31 immunostaining, clone JC70A). Note also the nearly complete CD31 staining of the cell surfaces of both intrasinusal SEVCs and the sinus-lining SEVC layer. A case of resorptive sinus histiocytosis (c, d cervical lymph node) exhibits abundant sinus histiocytes with vacuolated cytoplasm (c H&E staining); the hyperplastic SEVCs are clearly visible only after specific immunostaining, in this case, by a desmoplakin antibody (d clone DP-2.17). Bar 100 µm
Sinus histiocytosis, another reactive lymph node change that can develop in the draining area of chronic inflammations or tumors is characterized by expanded sinus filled with large histiocytes (“swollen macrophages”). Figure 18c shows the case of a particularly pronounced sinus histiocytosis with resorptive vacuolization of the sinus histiocytes. Whereas in routine light microscopy, SEVCs are difficult to recognize on the background of the prominent histiocytes (Fig. 18c), immunohistochemistry (here using desmoplakin as a SEVC marker) clearly highlights these cells, which again are hyperplastic and form an extended network spanning between the sinus histiocytes (Fig. 18d; cf. Schmelz et al. 1994). Since SEVCs appear to express VEGFR-3 (Fig. 16b), its ligand VEGF-C, which can be synthesized by tumors (Jackson et al. 2001), might be involved in the induction of SEVC hyperplasia. Increased numbers of SEVCs with prominent connexin 43 have been observed in lymph nodes involved in Langerhans cell histiocytosis and in malignant lymphomas (Krenács and Rosendaal 1995). In contrast, in monocytoid B-cell reaction occurring in the context of Piringer’s lymphadenitis, decreased numbers and interrupted arrangements of SEVCs have been recognized by monoclonal antibody Ki-M9 (Wacker 1994).
Tumors
Although the lymph node is generally known as a major site of lymphoid neoplasms and of metastatic tumor spread and growth (for a review, see Chambers et al. 2002), we should not overlook the existence of some specific tumors directly derived from non-lymphatic architectural cell types of the lymph node. All major reticulum-forming cell types seem to be able to give rise to certain tumors, which appear to maintain the synthesis of some cell-type-specific proteins and thus can be diagnostically characterized and related to the cell type of origin. As is frequently stated, the autochthonous tumors of the lymph node present a severe diagnostic problem, primarily because of the lack of reliable morphological properties. Thus, molecular markers should provide a highly desired armamentarium of potential value in the diagnoses of such tumors.
FDC tumors
FDCs, which form the three-dimensional multiform branching meshwork of the reticulum system of lymph follicles, including those of the lymph nodes, have been known for more than two decades to be able to transform into a special tumor subtype, classified as FDC tumors or sarcomas (Monda et al. 1986). Ultrastructurally, these tumors exhibit dendritic cell processes connected by scattered small but mature desmosomes. Immunohistochemically, FDC sarcomas are characterized by positive reactions for vimentin and desmoplakin and other desmosomal components, and with the advent of appropriate molecular markers, the frequency of diagnoses of these tumors has markedly increased (e.g., Perez-Ordonez et al. 1996; Chan et al. 1997; Chan et al. 2000; Sun et al. 2003; for a review, see Fonseca et al. 1998; see therein for further references). However, the molecular characterization of such tumors of mesenchymal cell origin, notably in lymph nodes, spleen, and dispersed lymphatic follicles, is still very much in its infancy, as these tumors represent typical examples in which morphological criteria or “broad”, i.e. non-selective, markers are simply not sufficient for a diagnosis justifiable on cell biological grounds (for discussion, see also S.W. Weiss et al. 1989; L.M. Weiss et al. 1990; Kawachi et al. 2002).
IDC tumors
These extremely rare neoplasms exhibit phenotypic features similar to those of IDCs, antigen-presenting cells of the T-cell system (Jaffe et al. 2001). Like their normal counterparts, these interdigitating dendritic cell sarcomas/tumors are characterized by S-100 protein. Ultrastructurally, they show interdigitating cell processes, but desmosomes are not present. Data on junctional markers are to our knowledge not yet available.
FRC tumors
FRCs outside of follicles are not well defined with respect to cell and molecular biological markers. Nevertheless, they appear to represent another kind of architectural and accessory cell that can give rise to special tumors, including metastatic tumors, originating in lymph nodes or in the spleen (for cases and detailed discussion, see Andriko et al. 1998; Martel et al. 2003).
CIRC tumors
CIRCs (Franke and Moll 1987; Gould et al. 1995; see also Doglioni et al. 1990) have been characterized as a subtype of fibroblastoid interstitial cells, i.e., FRCs that are located between the follicles and the sinus and that constitute the major cellular framework of the lymph node parenchyma. CIRCs have been identified as the cell type of origin of a special group of tumors, usually referred to as CIRC tumors or CIRC sarcomas. Following their first description by Gould et al. (1990), a series of publications have confirmed the existence of this tumor group and have added further valuable biological and clinical observations (e.g., Gould et al. 1995; Chan et al. 2000; Lucioni et al. 2003; Schuerfeld et al. 2003; Dong et al. 2008). In general, cytokeratin positivity, especially for keratins 8 and 18, and desmoplakin-negative reactions are at present the most valuable immunohistochemical characteristics of these genuine lymph node sarcomas, which include some rather aggressive forms.
SEVC-derived sarcomas
Impressed by the intense LYVE-1 immunostaining reaction in a single case of a dendritic-cell-like tumor, Krokowski et al. (2008) have recently proposed the existence of another distinct subtype of a dendritic lymph node cell sarcoma, which they have termed “sarcoma of sinus-lining cells”. The case described was characterized by the presence of CD31 and LYVE-1. Previously, Wacker (1994) had described a case of reticulosarcoma (reticulosis) of sinus wall cells. The series of molecular markers of SEVCs, notably those of the complexus adhaerens presented in this review, should now provide useful diagnostic reagents to identify and define this category of potential SEVC tumors.
Conclusions and perspectives
The molecular characterization of the morphological subforms of lining and intraluminal cells of the lymph node sinus now obviously provides sufficient data and markers to identify these cells, to subsume them under a collective term as “sinus endothelial/virgultar cells” (SEVCs), and to lay to rest the various hypotheses of a non-endothelial (e.g., monocytic, FRC) nature and origin of these cells (for controversial hypotheses and arguments, see Wacker 1994; Wacker et al. 1997; Gretz et al. 1997; Welsch et al. 1997; Crivellato and Mallardi 1998; Kaldjian et al. 2001; Willard-Mack 2006; and references cited therein; for a “historic” discussion, see also Raviola 1975). Clearly, the presence of an impressive ensemble of true endothelial differentiation markers establishes their endothelial or endothelium-derived nature and origin. The absence of any keratins and true desmosomes also apparently excludes a derivation from or relationship with FRCs (including CIRCs) or FDCs.
Our conclusion that the morphologically highly different lining and luminal meshwork cells of the SEVC system of the lymph node sinus are indeed endothelial and endothelial-cell-derived morphotypes is strongly supported by the findings that they constitutively contain characteristic vascular and lymphatic endothelial hallmark molecules such as factor-VIII-related antigen, CD31, VE-cadherin, claudin-5, JAM-A, LYVE-1, and VEGFR-3, together with the “special” component, desmoplakin. A similar though not identical combination of molecular markers (e.g., factor-VIII-related antigen, CD31, VE-cadherin, claudin-5, JAM-A, LYVE-1, VEGFR-3, and desmoplakin, but in addition ESAM-1 and high levels of podoplanin) is present in various other parts of the lymphatic vessel system, including several lymphatic capillaries (e.g., Schmelz et al. 1994; Baluk et al. 2007; Pfeiffer et al. 2008). Thus, despite being divided into two major morphotypes, SEVCs can be regarded as one distinct cell type, specifically as a special subtype of endothelial cells related to, but not completely identical with, lymphatic vessel endothelium. Therefore, as a corollary, one might consider the SEVC system of the lymph node sinus as a local and specific modification of the general lymphatic vasculature system.
An impressive morphological and molecular characteristic of the SEVC system is their frequent, variously sized, complex cell-cell junctions of a unique type, the complexus adhaerens, which combines typical molecules of three different types of cell-cell junctions, i.e., the transmembrane glycoprotein VE-cadherin, α- and β-catenin, p120 catenin, and afadin of adherens junctions, desmoplakin and plakoglobin of desmosomes, and the tight-junction-characteristic transmembrane proteins, claudin-5 and JAM-A, together with protein ZO-1, a plaque protein generally common to tight, adherens, and gap junctions. We have also identified and characterized an extended and tight ensheathing of the sinusal trabecular tissue and the extracellular reticular fibers, notably those with paracrystalline collagen bundles, by thin cytoplasmic processes of SEVCs, which again are “stitched” together with series of complexus adhaerentes. On the other hand, we have observed some impressive differences between the complexus adhaerens of the SEVC system and the adherens junctions of other endothelia, such as the abundance, in the former, of desmoplakin and the absence of appreciable amounts of the transmembrane glycoprotein N-cadherin and the plaque protein, p0071. We hypothesize that the SEVCs and their frequent and cell-type-specific complexus adhaerens junctions with their unique and complex ensemble of junctional molecules provide the architectural basis for major functions of the lymph node sinus; we have discussed above the compositional differences of the endothelial organisation in various parts of the lymph node in comparison with other parts of the vascular system.
Indeed, lymphocytes, monocytes, notably macrophages and mast cells, plasma cells, and polymorphonuclear leukocytes can all be seen within the sinus confinements, but they do not markedly contribute to the basic sinus architecture. However, as suggested by the expanded surface of the SEVC system and the presence of adhesive surface molecules on SEVCs, all these cells probably interact in different ways with the SEVC system, as most probably do malignant cells from solid tumors that have found their way into lymph node sinus during early lymphatic dissemination. The elucidation of the specific mechanisms of these interactions will be extremely important in future studies. The molecular markers and interactions demonstrated have enhanced our understanding of lymph node functions and should provide a better basis for future research in immunology and in pathology, including improved detection and classification of autochthonous lymph node tumors and deeper insight into the mechanisms of early stages of lymph node metastases.
Notes
As this is a Latin word, we retain the correct plural form throughout this article.
As the meaning of the Latin words “rete” and “reticulum” is obviously restricted to one-layered two-dimensional “net” structures, we here omit the use of the term “retothelium”, originally coined by Roulet (1954) and meant to include diverse reticular cell types of the lymphatic sinus and pulpa. Instead, we shall refer to the entirety of the three-dimensional meshwork of cells and cell processes extending throughout the sinus lumen as a “virgultum”, i.e., a space filled with elongated structures resembling bushes and brush forming a thicket or shrubbery of cells and cell projections, with many branches and without a preferential order. Consequently, we refer to the endothelial monolayer as “sinus-lining endothelium” and to the intraluminal three-dimensional bushwork of endothelium-type cell structures as the “virgultum”.
References
Ahrens T, Lambert M, Pertz O, Sasaki T, Schulthess T, Mege RM, Timpl R, Engel J (2003) Homoassociation of VE-cadherin follows a mechanism common to “classical” cadherins. J Mol Biol 325:733–742
Aijaz S, Balda MS, Matter K (2006) Tight junctions: molecular architecture and function. Int Rev Cytol 248:261–298
Akat K, Bleck CK, Lee YM, Haselmann-Weiss U, Kartenbeck J (2008) Characterization of a novel type of adherens junction in meningiomas and the derived cell line HBL-52. Cell Tissue Res 331:401–412
Akazaki K (1953) Tumors of the reticulo-endothelial system. Acta Pathol Jpn 3:24–43
Alexander JS, Jackson SA, Chaney E, Kevil CG, Haselton FR (1998) The role of cadherin endocytosis in endothelial barrier regulation: involvement of protein kinase C and actin-cadherin interactions. Inflammation 22:419–433
Al-Rawi MA, Mansel RE, Jiang WG (2005) Lymphangiogenesis and its role in cancer. Histol Histopathol 20:283–298
Alitalo K, Tammela T, Petrova TV (2005) Lymphangiogenesis in development and human disease. Nature 438:946–953
Allport JR, Ding H, Collins T, Gerritsen ME, Luscinskas FW (1997) Endothelial-dependent mechanisms regulate leukocyte transmigration: a process involving the proteasome and disruption of the vascular endothelial-cadherin complex at endothelial cell-to-cell junctions. J Exp Med 186:517–527
Anastasiadis PZ, Reynolds AB (2000) The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci 113:1319–1334
Andrian UH von, Mempel TR (2003) Homing and cellular traffic in lymph nodes. Nat Rev Immunol 3:867–878
Andriko JW, Kaldjian EP, Tsokos M, Abbondanzo SL, Jaffe ES (1998) Reticulum cell neoplasms of lymph nodes: a clinicopathologic study of 11 cases with recognition of a new subtype derived from fibroblastic reticular cells. Am J Surg Pathol 22:1048–1058
Aschoff L (1924) Das reticulo-endotheliale System. Ergeb Inn Med Kinderheilkd 26:1–118
Aurrand-Lions M, Duncan L, Ballestrem C, Imhof BA (2001) JAM-2, a novel immunoglobulin superfamily molecule, expressed by endothelial and lymphatic cells. J Biol Chem 276:2733–2741
Bajénoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN (2006) Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity 25:989–1001
Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM (2007) Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med 204:2349–2362
Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG (1999) LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol 144:789–801
Birk DE, Trelstad RL (1986) Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J Cell Biol 103:231–240
Borrmann CM, Grund C, Kuhn C, Hofmann I, Pieperhoff S, Franke WW (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. Eur J Cell Biol 85:469–485
Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154:385–394
Breviario F, Caveda L, Corada M, Martin-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E (1995) Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol 15:1229–1239
Burgio VL, Zupo S, Roncella S, Zocchi M, Ruco LP, Baroni CD (1994) Characterization of EN4 monoclonal antibody: a reagent with CD31 specificity. Clin Exp Immunol 96:170–176
Burns AR, Bowden RA, MacDonell SD, Walker DC, Odebunmi TO, Donnachie EM, Simon SI, Entman ML, Smith CW (2000) Analysis of tight junctions during neutrophil transendothelial migration. J Cell Sci 113:45–57
Calkins CC, Hoepner BL, Law CM, Novak MR, Setzer SV, Hatzfeld M, Kowalczyk AP (2003) The armadillo family protein p0071 is a VE-cadherin- and desmoplakin-binding protein. J Biol Chem 278:1774–1783
Canty EG, Lu Y, Meadows RS, Shaw MK, Holmes DF, Kadler KE (2004) Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J Cell Biol 165:553–563
Canty EG, Starborg T, Lu Y, Humphries SM, Holmes DF, Meadows RS, Huffman A, O’Toole ET, Kadler KE (2006) Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J Biol Chem 281:38592–38598
Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oostuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, Ruiter MC de, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147–157
Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, Bianco P, Wolburg H, Moore R, Oreda B, Kemler R, Dejana E (2003) The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol 162:1111–1122
Caveda L, Martin-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, Dejana E (1996) Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin). J Clin Invest 98:886–893
Chambers AF, Groom AC, MacDonald IC (2002) Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2:563–572
Chan AC, Serrano-Olmo J, Erlandson RA, Rosai J (2000) Cytokeratin-positive malignant tumors with reticulum cell morphology: a subtype of fibroblastic reticulum cell neoplasm? Am J Surg Pathol 24:107–116
Chan JK, Fletcher CD, Nayler SJ, Cooper K (1997) Follicular dendritic cell sarcoma. Clinicopathologic analysis of 17 cases suggesting a malignant potential higher than currently recognized. Cancer 79:294–313
Chikwava K, Jaffe R (2004) Langerin (CD207) staining in normal pediatric tissues, reactive lymph nodes, and childhood histiocytic disorders. Pediatr Dev Pathol 7:607–614
Compton CC, Raviola E (1985) Structure of the sinus-lining cells in the popliteal lymph node of the rabbit. Anat Rec 212:408–423
Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E (1999) Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA 96:9815–9820
Corada M, Liao F, Lindgren M, Lampugnani MG, Breviario F, Frank R, Muller WA, Hicklin DJ, Bohlen P, Dejana E (2001) Monoclonal antibodies directed to different regions of vascular endothelial cadherin extracellular domain affect adhesion and clustering of the protein and modulate endothelial permeability. Blood 97:1679–1684
Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO (1986) Plakoglobin: a protein common to different kinds of intercellular adhering junctions. Cell 46:1063–1073
Crivellato E, Mallardi F (1998) The sinus endothelial cell architecture in the mouse lymph node. Structural peculiarities and close correlation with the fibroblastic reticular cells. J Submicrosc Cytol Pathol 30:495–502
Danilov SM, Muzykantov VR, Martynov AV, Atochina EN, Sakharov IY, Trakht IN, Smirnov VN (1991) Lung is the target organ for a monoclonal antibody to angiotensin-converting enzyme. Lab Invest 64:118–124
Dejana E (1996) Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest 98:1949–1953
Dejana E (2004) Endothelial cell-cell junctions: happy together. Nat Rev Mol Cell Biol 5:261–270
Dejana E, Bazzoni G, Lampugnani MG (1999) Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res 252:13–19
Dejana E, Lampugnani MG, Martinez-Estrada O, Bazzoni G (2000) The molecular organization of endothelial junctions and their functional role in vascular morphogenesis and permeability. Int J Dev Biol 44:743–748
Del Maschio A, Zanetti A, Corada M, Rival Y, Ruco L, Lampugnani MG, Dejana E (1996) Polymorphonuclear leukocyte adhesion triggers the disorganization of endothelial cell-to-cell adherens junctions. J Cell Biol 135:497–510
Doglioni C, Dell’Orto P, Zanetti G, Iuzzolino P, Coggi G, Viale G (1990) Cytokeratin-immunoreactive cells of human lymph nodes and spleen in normal and pathological conditions. An immunocytochemical study. Virchows Arch [A] 416:479–490
Dong YC, Wu B, Sheng Z, Wang JD, Zhou HB, Zhou XJ (2008) Cytokeratin-positive interstitial reticulum cell tumors of lymph nodes: a case report and review of literature. Chin Med J (Engl) 121:658–663
Drinker CK, Wislocki GB, Field ME (1933) The structure of the sinuses of the lymph nodes. Anat Rec 56:261–273
Drinker CK, Field ME, Ward HK (1934) The filtering capacitiy of lymph nodes. J Exp Med 59:393–405
Duff SE, Li C, Garland JM, Kumar S (2003) CD105 is important for angiogenesis: evidence and potential applications. FASEB J 17:984–992
Düllmann J, Van Damme EJ, Peumans WJ, Ziesenitz M, Schumacher U (2002) Lectin histochemistry of the rat lymph node: visualisation of stroma, blood vessels, sinuses, and macrophages. A contribution to the concept of an immune accessory role of sinus-lining endothelia. Acta Histochem 104:77–83
Ebata N, Nodasaka Y, Sawa Y, Yamaoka Y, Makino S, Totsuka Y, Yoshida S (2001a) Desmoplakin as a specific marker of lymphatic vessels. Microvasc Res 61:40–48
Ebata N, Sawa Y, Nodasaka Y, Yamaoka Y, Yoshida S, Totsuka Y (2001b) Immunoelectron microscopic study of PECAM-1 expression on lymphatic endothelium of the human tongue. Tissue Cell 33:211–218
Ebnet K (2008) Organization of multiprotein complexes at cell-cell junctions. Histochem Cell Biol 130:1–20
Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, Suzuki A, Imhof BA, Vestweber D (2003) The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci 116:3879–3891
Farr AG, Cho Y, De Bruyn PP (1980) The structure of the sinus wall of the lymph node relative to its endocytic properties and transmural cell passage. Am J Anat 157:265–284
Ferber A, Yaen C, Sarmiento E, Martinez J (2002) An octapeptide in the juxtamembrane domain of VE-cadherin is important for p120ctn binding and cell proliferation. Exp Cell Res 274:35–44
Ferreri DM, Vincent PA (2008) Signaling to and through endothelial adherens junction. In: LaFlamme SE, Kowalczyk AP (eds) Cell junctions. Adhesion, development, and disease. WILEY-VCH, Weinheim, pp 169–195
Fiedler U, Christian S, Koidl S, Kerjaschki D, Emmett MS, Bates DO, Christofori G, Augustin HG (2006) The sialomucin CD34 is a marker of lymphatic endothelial cells in human tumors. Am J Pathol 168:1045–1053
Fonseca R, Yamakawa M, Nakamura S, Heerde P van, Miettinen M, Shek TW, Myhre JO, Rousselet MC, Tefferi A (1998) Follicular dendritic cell sarcoma and interdigitating reticulum cell sarcoma: a review. Am J Hematol 59:161–167
Forkert PG, Thliveris JA, Bertalanffy FD (1977) Structure of sinuses in the human lymph node. Cell Tissue Res 183:115–130
Franke WW, Moll R (1987) Cytoskeletal components of lymphoid organs. I. Synthesis of cytokeratins 8 and 18 and desmin in subpopulations of extrafollicular reticulum cells of human lymph nodes, tonsils, and spleen. Differentiation 36:145–163
Franke WW, Schmid E, Osborn M, Weber K (1979) Intermediate-sized filaments of human endothelial cells. J Cell Biol 81:570–580
Franke WW, Schmid E, Grund C, Müller H, Engelbrecht I, Moll R, Stadler J, Jarasch E-D (1981) Antibodies to high molecular weight polypeptides of desmosomes: specific localization of a class of junctional proteins in cells and tissues. Differentiation 20:217–241
Franke WW, Moll R, Schiller DL, Schmid E, Kartenbeck J, Müller H (1982) Desmoplakins of epithelial and myocardial desmosomes are immunologically and biochemically related. Differentiation 23:115–127
Franke WW, Kapprell HP, Cowin P (1987) Immunolocalization of plakoglobin in endothelial junctions: identification as a special type of zonulae adhaerentes. Biol Cell 59:205–218
Franke WW, Cowin P, Grund C, Kuhn C, Kapprell HP (1988) The endothelial junction. The plaque and its components. In: Simionescu N, Simionescu M (eds) Endothelial cell biology in health and disease. Plenum, New York, pp 147–166
Franke WW, Goldschmidt MD, Zimbelmann R, Mueller HM, Schiller DL, Cowin P (1989a) Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci USA 86:4027–4031
Franke WW, Jahn L, Knapp AC (1989b) Cytokeratins and desmosomal proteins in certain epithelioid and nonepithelial cells. In: Osborn M, Weber K (eds) Cytoskeletal proteins in tumor diagnosis. Current communications in molecular biology. Cold Spring Harbour Laboratory, Cold Spring Harbor, pp 151–172
Franke WW, Koch PJ, Schäfer S, Heid HW, Troyanovsky SM, Moll I, Moll R (1994) The desmosome and the syndesmos: cell junctions in normal development and in malignancy. Princess Takamatsu Symp 24:14–27
Franke WW, Borrmann CM, Grund C, Pieperhoff S (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. Eur J Cell Biol 85:69–82
Fujita T, Miyoshi M, Murakami T (1972) Scanning electron microscope observation of the dog mesenteric lymph node. Z Zellforsch Mikrosk Anat 133:147–162
Gallicano GI, Bauer C, Fuchs E (2001) Rescuing desmoplakin function in extra-embryonic ectoderm reveals the importance of this protein in embryonic heart, neuroepithelium, skin and vasculature. Development 128:929–941
Gavard J, Gutkind JS (2006) VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol 8:1223–1234
Goossens S, Janssens B, Bonne S, De Rycke R, Braet F, Hengel J van, Roy F van (2007) A unique and specific interaction between alphaT-catenin and plakophilin-2 in the area composita, the mixed-type junctional structure of cardiac intercalated discs. J Cell Sci 120:2126–2136
Gory-Fauré S, Prandini MH, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P (1999) Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126:2093–2102
Gould VE, Warren WH, Faber LP, Kuhn C, Franke WW (1990) Malignant cells of epithelial phenotype limited to thoracic lymph nodes. Eur J Cancer 26:1121–1126
Gould VE, Bloom KJ, Franke WW, Warren WH, Moll R (1995) Increased numbers of cytokeratin-positive interstitial reticulum cells (CIRC) in reactive, inflammatory and neoplastic lymphadenopathies: hyperplasia or induced expression? Virchows Arch 425:617–629
Gretz JE, Anderson AO, Shaw S (1997) Cords, channels, corridors and conduits: critical architectural elements facilitating cell interactions in the lymph node cortex. Immunol Rev 156:11–24
Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S (2000) Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med 192:1425–1440
Grossmann KS, Grund C, Huelsken J, Behrend M, Erdmann B, Franke WW, Birchmeier W (2004) Requirement of plakophilin 2 for heart morphogenesis and cardiac junction formation. J Cell Biol 167:149–160
Hämmerling B (2004) Molekulare Charakterisierung der verschiedenen Zell-Zell-Verbindungsstrukturen (“Junctions”) in Lymphknoten höherer Säugetiere. [Molecular characterization of the diverse cell-cell-junctions in lymph nodes of higher mammals]. M.D. Thesis. Faculty of Medicine, Ruprechts-Karls-University, Heidelberg, Germany
Hämmerling B, Grund C, Boda-Heggemann J, Moll R, Franke WW (2006) The complexus adhaerens of mammalian lymphatic endothelia revisited: a junction even more complex than hitherto thought. Cell Tissue Res 324:55–67
Haselton FR, Heimark RL (1997) Role of cadherins 5 and 13 in the aortic endothelial barrier. J Cell Physiol 171:243–251
Hatzfeld M (2005) The p120 family of cell adhesion molecules. Eur J Cell Biol 84:205–214
Hatzfeld M (2007) Plakophilins: multifunctional proteins or just regulators of desmosomal adhesion? Biochim Biophys Acta 1773:69–77
Hatzfeld M, Nachtsheim C (1996) Cloning and characterization of a new armadillo family member, p0071, associated with the junctional plaque: evidence for a subfamily of closely related proteins. J Cell Sci 109:2767–2778
Hayakawa M, Kobayashi M, Hoshino T (1990) Microfibrils: a constitutive component of reticular fibers in the mouse lymph node. Cell Tissue Res 262:199–201
Hayes H, Kossmann E, Wilson E, Meininger C, Zawieja D (2003) Development and characterization of endothelial cells from rat microlymphatics. Lymphat Res Biol 1:101–119
Heid HW, Schmidt A, Zimbelmann R, Schäfer S, Winter-Simanowski S, Stumpp S, Keith M, Figge U, Schnölzer M, Franke WW (1994) Cell type-specific desmosomal plaque proteins of the plakoglobin family: plakophilin 1 (band 6 protein). Differentiation 58:113–131
Hinz B, Pittet P, Smith-Clerc J, Chaponnier C, Meister JJ (2004) Myofibroblast development is characterized by specific cell-cell adherens junctions. Mol Biol Cell 15:4310–4320
Hofmann I, Schlechter T, Kuhn C, Hergt M, Franke WW (2008) Protein p0071, an armadillo plaque protein that characterizes a specific subtype of adherens junctions and their functions. J Cell Sci, in press
Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D (1999) Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci 112:1915–1923
Horikawa K, Radice G, Takeichi M, Chisaka O (1999) Adhesive subdivisions intrinsic to the epithelial somites. Dev Biol 215:182–189
Irjala H, Elima K, Johansson EL, Merinen M, Kontula K, Alanen K, Grenman R, Salmi M, Jalkanen S (2003) The same endothelial receptor controls lymphocyte traffic both in vascular and lymphatic vessels. Eur J Immunol 33:815–824
Iyer S, Ferreri DM, DeCocco NC, Minnear FL, Vincent PA (2004) VE-cadherin-p120 interaction is required for maintenance of endothelial barrier function. Am J Physiol Lung Cell Mol Physiol 286:L1143–L1153
Jackson DE (2003) The unfolding tale of PECAM-1. FEBS Lett 540:7–14
Jackson DG, Prevo R, Clasper S, Banerji S (2001) LYVE-1, the lymphatic system and tumor lymphangiogenesis. Trends Immunol 22:317–321
Jaffe ES, Harris NL, Stein H, Vardiman JW (2001) Pathology and genetics of tumours of haematopoietic and lymphoid tissues. World Health Organization Classification of Tumours. IARC Press, Lyon
Jaggi M, Wheelock MJ, Johnson KR (2002) Differential displacement of classical cadherins by VE-cadherin. Cell Commun Adhes 9:103–115
Johnson-Léger C, Aurrand-Lions M, Imhof BA (2000) The parting of the endothelium: miracle, or simply a junctional affair? J Cell Sci 113:921–933
Junt T, Moseman EA, Iannacone M, Massberg S, Lang PA, Boes M, Fink K, Henrickson SE, Shayakhmetov DM, Di Paolo NC, Rooijen N van, Mempel TR, Whelan SP, Andrian UH von (2007) Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450:110–114
Kaipainen A, Korhonen J, Mustonen T, Hinsbergh VW van, Fang GH, Dumont D, Breitman M, Alitalo K (1995) Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA 92:3566–3570
Kaiserling E, Xiao JC, Adam A, Ruck P (1998) Der Lymphknotensinus als Leitungssystem. Morphologische und immunhistochemische Befunde. In: Kaiserling E, Kröber SM, Ruck P (eds) Lymphologica 1997 Year Book. Kagerer Kommunikation, Bonn, pp 37–43
Kaldjian EP, Gretz JE, Anderson AO, Shi Y, Shaw S (2001) Spatial and molecular organization of lymph node T cell cortex: a labyrinthine cavity bounded by an epithelium-like monolayer of fibroblastic reticular cells anchored to basement membrane-like extracellular matrix. Int Immunol 13:1243–1253
Kartenbeck J, Schwechheimer K, Moll R, Franke WW (1984) Attachment of vimentin filaments to desmosomal plaques in human meningiomal cells and arachnoidal tissue. J Cell Biol 98:1072–1081
Kawachi K, Nakatani Y, Inayama Y, Kawano N, Toda N, Misugi K (2002) Interdigitating dendritic cell sarcoma of the spleen: report of a case with a review of the literature. Am J Surg Pathol 26:530–537
Kawaguchi J, Azuma Y, Hoshi K, Kii I, Takeshita S, Ohta T, Ozawa H, Takeichi M, Chisaka O, Kudo A (2001a) Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. J Bone Miner Res 16:1265–1271
Kawaguchi J, Kii I, Sugiyama Y, Takeshita S, Kudo A (2001b) The transition of cadherin expression in osteoblast differentiation from mesenchymal cells: consistent expression of cadherin-11 in osteoblast lineage. J Bone Miner Res 16:260–269
Kevil CG, Payne DK, Mire E, Alexander JS (1998) Vascular permeability factor/vascular endothelial cell growth factor-mediated permeability occurs through disorganization of endothelial junctional proteins. J Biol Chem 273:15099–15103
Koch PJ, Walsh MJ, Schmelz M, Goldschmidt MD, Zimbelmann R, Franke WW (1990) Identification of desmoglein, a constitutive desmosomal glycoprotein, as a member of the cadherin family of cell adhesion molecules. Eur J Cell Biol 53:1–12
Koeser J, Troyanovsky SM, Grund C, Franke WW (2003) De novo formation of desmosomes in cultured cells upon transfection of genes encoding specific desmosomal components. Exp Cell Res 285:114–130
Kowalczyk AP, Navarro P, Dejana E, Bornslaeger EA, Green KJ, Kopp DS, Borgwardt JE (1998) VE-cadherin and desmoplakin are assembled into dermal microvascular endothelial intercellular junctions: a pivotal role for plakoglobin in the recruitment of desmoplakin to intercellular junctions. J Cell Sci 111:3045–3057
Kramer RH, Rosen SD, McDonald KA (1988) Basement-membrane components associated with the extracellular matrix of the lymph node. Cell Tissue Res 252:367–375
Krenács T, Rosendaal M (1995) Immunohistological detection of gap junctions in human lymphoid tissue: connexin43 in follicular dendritic and lymphoendothelial cells. J Histochem Cytochem 43:1125–1137
Krokowski M, Merz H, Thorns C, Bernd HW, Schade U, Le TA, Diebold J, Feller AC (2008) Sarcoma of follicular dendritic cells with features of sinus lining cells—a new subtype of reticulum cell sarcoma? Virchows Arch 452:565–570
Kubo H, Alitalo K (2003) The bloody fate of endothelial stem cells. Genes Dev 17:322–329
Lambeng N, Wallez Y, Rampon C, Cand F, Christe G, Gulino-Debrac D, Vilgrain I, Huber P (2005) Vascular endothelial-cadherin tyrosine phosphorylation in angiogenic and quiescent adult tissues. Circ Res 96:384–391
Lampugnani MG, Dejana E (1997) Interendothelial junctions: structure, signalling and functional roles. Curr Opin Cell Biol 9:674–682
Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E (1995) The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, beta-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin). J Cell Biol 129:203–217
Lampugnani MG, Zanetti A, Breviario F, Balconi G, Orsenigo F, Corada M, Spagnuolo R, Betson M, Braga V, Dejana E (2002) VE-cadherin regulates endothelial actin activating Rac and increasing membrane association of Tiam. Mol Biol Cell 13:1175–1189
Lucioni M, Boveri E, Rosso R, Benazzo M, Necchi V, Danova M, Incardona P, Franco C, Viglio A, Riboni R, Lazzarino M, Magrini U, Canevari A, Paulli M (2003) Lymph node reticulum cell neoplasm with progression into cytokeratin-positive interstitial reticulum cell (CIRC) sarcoma: a case study. Histopathology 43:583–591
Luo Y, Radice GL (2005) N-cadherin acts upstream of VE-cadherin in controlling vascular morphogenesis. J Cell Biol 169:29–34
Luo Y, Kostetskii I, Radice GL (2005) N-cadherin is not essential for limb mesenchymal chondrogenesis. Dev Dyn 232:336–344
MacCalman CD, Furth EE, Omigbodun A, Bronner M, Coutifaris C, Strauss JF III (1996) Regulated expression of cadherin-11 in human epithelial cells: a role for cadherin-11 in trophoblast-endometrium interactions? Dev Dyn 206:201–211
Mandell KJ, Parkos CA (2005) The JAM family of proteins. Adv Drug Deliv Rev 57:857–867
Martel M, Sarli D, Colecchia M, Coppa J, Romito R, Schiavo M, Mazzaferro V, Rosai J (2003) Fibroblastic reticular cell tumor of the spleen: report of a case and review of the entity. Hum Pathol 34:954–957
Martinez-Pomares L, Gordon S (2007) Antigen presentation the macrophage way. Cell 131:641–643
McCrea PD, Park JI (2007) Developmental functions of the P120-catenin sub-family. Biochim Biophys Acta 1773:17–33
Mertens C, Kuhn C, Franke WW (1996) Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol 135:1009–1025
Mertens C, Kuhn C, Moll R, Schwetlick I, Franke WW (1999) Desmosomal plakophilin 2 as a differentiation marker in normal and malignant tissues. Differentiation 64:277–290
Mertens C, Hofmann I, Wang Z, Teichmann M, Sepehri CS, Schnölzer M, Franke WW (2001) Nuclear particles containing RNA polymerase III complexes associated with the junctional plaque protein plakophilin 2. Proc Natl Acad Sci USA 98:7795–7800
Middel P, Patterson BW, Ophemert I van, Wacker HH, Parwaresch MR, Radzun HJ, Zschunke F (2002) Cloning of a complementary DNA encoding the unique dendritic cell antigen Ki-M9. Cell Tissue Res 307:347–355
Moe RE (1963) Fine structure of the reticulum and sinuses of lymph nodes. Am J Anat 112:311–335
Moll R, Cowin P, Kapprell HP, Franke WW (1986) Desmosomal proteins: new markers for identification and classification of tumors. Lab Invest 54:4–25
Moll R, Holzhausen HJ, Mennel HD, Kuhn C, Baumann R, Taege C, Franke WW (2006) The cardiac isoform of alpha-actin in regenerating and atrophic skeletal muscle, myopathies and rhabdomyomatous tumors: an immunohistochemical study using monoclonal antibodies. Virchows Arch 449:175–191
Monda L, Warnke R, Rosai J (1986) A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol 122:562–572
Müller J, Tvrdík D, Dvorák R, Djaborkhel R, Mandys V, Bednár B, Raska I, Lojda Z (2000) Expression of beta-catenins and cadherins by follicular dendritic cells in human lymph nodes. Acta Histochem 102:369–380
Navarro P, Caveda L, Breviario F, Mandoteanu I, Lampugnani MG, Dejana E (1995) Catenin-dependent and -independent functions of vascular endothelial cadherin. J Biol Chem 270:30965–30972
Navarro P, Ruco L, Dejana E (1998) Differential localization of VE- and N-cadherins in human endothelial cells: VE-cadherin competes with N-cadherin for junctional localization. J Cell Biol 140:1475–1484
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660
Nuber UA, Schäfer S, Schmidt A, Koch PJ, Franke WW (1995) The widespread human desmocollin Dsc2 and tissue-specific patterns of synthesis of various desmocollin subtypes. Eur J Cell Biol 66:69–74
Oxford EM, Musa H, Maass K, Coombs W, Taffet SM, Delmar M (2007) Connexin43 remodeling caused by inhibition of plakophilin-2 expression in cardiac cells. Circ Res 101:703–711
Perez-Ordonez B, Erlandson RA, Rosai J (1996) Follicular dendritic cell tumor: report of 13 additional cases of a distinctive entity. Am J Surg Pathol 20:944–955
Pfeiffer F, Kumar V, Butz S, Vestweber D, Imhof BA, Stein JV, Engelhardt B (2008) Distinct molecular composition of blood and lymphatic vascular endothelial cell junctions establishes specific functional barriers within the peripheral lymph node. Eur J Immunol 38:2142–2155
Phan TG, Grigorova I, Okada T, Cyster JG (2007) Subcapsular encounter and complement-dependent transport of immune complexes by lymph node B cells. Nat Immunol 8:992–1000
Pieperhoff S, Schumacher H, Franke WW (2008) The area composita of adhering junctions connecting heart muscle cells of vertebrates. V. The importance of plakophilin-2 demonstrated by small interference RNA-mediated knockdown in cultured rat cardiomyocytes. Eur J Cell Biol 87:399–411
Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M (2002) Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci USA 99:16069–16074
Prevo R, Banerji S, Ni J, Jackson DG (2004) Rapid plasma membrane-endosomal trafficking of the lymph node sinus and high endothelial venule scavenger receptor/homing receptor stabilin-1 (FEEL-1/CLEVER-1). J Biol Chem 279:52580–52592
Rampon C, Prandini MH, Bouillot S, Pointu H, Tillet E, Frank R, Vernet M, Huber P (2005) Protocadherin 12 (VE-cadherin 2) is expressed in endothelial, trophoblast, and mesangial cells. Exp Cell Res 302:48–60
Ratcliffe MJ, Smales C, Staddon JM (1999) Dephosphorylation of the catenins p120 and p100 in endothelial cells in response to inflammatory stimuli. Biochem J 338:471–478
Raviola E (1975) Lymph nodes. In: Bloom W, Fawcett DW (eds) A textbook of histology. Saunders, Philadelphia, pp 471–486
Reilly JT, Nash JR, Mackie MJ, McVerry BA (1985) Distribution of fibronectin and laminin in normal and pathological lymphoid tissue. J Clin Pathol 38:849–854
Richardson SH, Starborg T, Lu Y, Humphries SM, Meadows RS, Kadler KE (2007) Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell-cell junctions. Mol Cell Biol 27:6218–6228
Roulet FC (1954) Differential diagnosis of reticulocytic proliferation in the lymph nodes. Bibl Paediatr 58:706–721
Ruco LP, Pomponi D, Pigott R, Gearing AJ, Baiocchini A, Baroni CD (1992) Expression and cell distribution of the intercellular adhesion molecule, vascular cell adhesion molecule, endothelial leukocyte adhesion molecule, and endothelial cell adhesion molecule (CD31) in reactive human lymph nodes and in Hodgkin’s disease. Am J Pathol 140:1337–1344
Sainte-Marie G, Peng FS (1986) Diffusion of a lymph-carried antigen in the fiber network of the lymph node of the rat. Cell Tissue Res 245:481–486
Sakuma H, Kasajima T, Imai Y, Kojima M (1981) An electron microscopic study on the reticuloendothelial cells in the lymph nodes. Acta Pathol Jpn 31:449–472
Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B (1992) Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci 102:7–17
Schacht V, Dadras SS, Johnson LA, Jackson DG, Hong YK, Detmar M (2005) Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 166:913–921
Schmelz M, Franke WW (1993) Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells: the syndesmos connecting retothelial cells of lymph nodes. Eur J Cell Biol 61:274–289
Schmelz M, Moll R, Franke WW (1990) A new type of intercellular junction: desmosomal proteins in the extended junctions of certain endothelial cells of the lymphatic systems. Cell Biol Int Rep 14:54
Schmelz M, Moll R, Kuhn C, Franke WW (1994) Complexus adhaerentes, a new group of desmoplakin-containing junctions in endothelial cells. II. Different types of lymphatic vessels. Differentiation 57:97–117
Schmidt A, Jäger S (2005) Plakophilins—hard work in the desmosome, recreation in the nucleus? Eur J Cell Biol 84:189–204
Schuerfeld K, Lazzi S, De Santi MM, Gozzetti A, Leoncini L, Pileri SA (2003) Cytokeratin-positive interstitial cell neoplasm: a case report and classification issues. Histopathology 43:491–494
Schulze C, Firth JA (1993) Immunohistochemical localization of adherens junction components in blood-brain barrier microvessels of the rat. J Cell Sci 104:773–782
Shaw SK, Bamba PS, Perkins BN, Luscinskas FW (2001) Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J Immunol 167:2323–2330
Shibata T, Ochiai A, Gotoh M, Machinami R, Hirohashi S (1996) Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer Lett 99:147–153
Sievers E (2009) Sinusendothel-/Virgultumzellen in normalen und pathologisch veränderten menschlichen Lymphknoten. Morphologische Befunde und immunhistochemische Markerprofile. [Sinus endothelial/virgultar cells in normal and pathologically altered human lymph nodes]. M.D. Thesis. Faculty of Medicine, Philipps-University, Marburg, Germany
Simionescu N, Simionescu M (1988) Endothelial cell biology in health and disease. Plenum, New York
Simonneau L, Thiery JP (1998) The mesenchymal cadherin-11 is expressed in restricted sites during the ontogeny of the rat brain in modes suggesting novel functions. Cell Adhes Commun 6:431–450
Simonneau L, Kitagawa M, Suzuki S, Thiery JP (1995) Cadherin 11 expression marks the mesenchymal phenotype: towards new functions for cadherins? Cell Adhes Commun 3:115–130
Singer II, Tian M, Wickham LA, Lin J, Matheravidathu SS, Forrest MJ, Mandala S, Quackenbush EJ (2005) Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol 175:7151–7161
Sixt M, Kanazawa N, Selg M, Samson T, Roos G, Reinhardt DP, Pabst R, Lutz MB, Sorokin L (2005) The conduit system transports soluble antigens from the afferent lymph to resident dendritic cells in the T cell area of the lymph node. Immunity 22:19–29
Sleeman JP, Krishnan J, Kirkin V, Baumann P (2001) Markers for the lymphatic endothelium: in search of the holy grail? Microsc Res Tech 55:61–69
Smedsrød B (2004) Clearance function of scavenger endothelial cells. Comp Hepatol 3 (Suppl 1):S22
Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K (2002) Lymphangiogenesis and cancer metastasis. Nat Rev Cancer 2:573–583
Sun X, Chang KC, Abruzzo LV, Lai R, Younes A, Jones D (2003) Epidermal growth factor receptor expression in follicular dendritic cells: a shared feature of follicular dendritic cell sarcoma and Castleman’s disease. Hum Pathol 34:835–840
Suzuki K, Kusumoto H, Deyashiki Y, Nishioka J, Maruyama I, Zushi M, Kawahara S, Honda G, Yamamoto S, Horiguchi S (1987) Structure and expression of human thrombomodulin, a thrombin receptor on endothelium acting as a cofactor for protein C activation. EMBO J 6:1891–1897
Takai Y, Nakanishi H (2003) Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci 116:17–27
Telo’ P, Breviario F, Huber P, Panzeri C, Dejana E (1998) Identification of a novel cadherin (vascular endothelial cadherin-2) located at intercellular junctions in endothelial cells. J Biol Chem 273:17565–17572
Tew JG, Kosco MH, Burton GF, Szakal AK (1990) Follicular dendritic cells as accessory cells. Immunol Rev 117:185–211
Tew JG, Wu J, Fakher M, Szakal AK, Qin D (2001) Follicular dendritic cells: beyond the necessity of T-cell help. Trends Immunol 22:361–367
Ueki T, Iwasawa K, Ishikawa H, Sawa Y (2008) Expression of junctional adhesion molecules on the human lymphatic endothelium. Microvasc Res 75:269–278
Ukropec JA, Hollinger MK, Salva SM, Woolkalis MJ (2000) SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J Biol Chem 275:5983–5986
Ushiki T, Ohtani O, Abe K (1995) Scanning electron microscopic studies of reticular framework in the rat mesenteric lymph node. Anat Rec 241:113–122
Valiron O, Chevrier V, Usson Y, Breviario F, Job D, Dejana E (1996) Desmoplakin expression and organization at human umbilical vein endothelial cell-to-cell junctions. J Cell Sci 109:2141–2149
Vittet D, Buchou T, Schweitzer A, Dejana E, Huber P (1997) Targeted null-mutation in the vascular endothelial-cadherin gene impairs the organization of vascular-like structures in embryoid bodies. Proc Natl Acad Sci USA 94:6273–6278
Wacker HH (1994) Sinus lining cells. Immune accessory cells of lymph node sinuses. Veröff Pathol 143:1–217
Wacker HH, Frahm SO, Heidebrecht HJ, Parwaresch R (1997) Sinus-lining cells of the lymph nodes recognized as a dendritic cell type by the new monoclonal antibody Ki-M9. Am J Pathol 151:423–434
Wei SH, Rosen H, Matheu MP, Sanna MG, Wang SK, Jo E, Wong CH, Parker I, Cahalan MD (2005) Sphingosine 1-phosphate type 1 receptor agonism inhibits transendothelial migration of medullary T cells to lymphatic sinuses. Nat Immunol 6:1228–1235
Weiss LM, Berry GJ, Dorfman RF, Banks P, Kaiserling E, Curtis J, Rosai J, Warnke RA (1990) Spindle cell neoplasms of lymph nodes of probable reticulum cell lineage. True reticulum cell sarcoma? Am J Surg Pathol 14:405–414
Weiss SW, Gnepp DR, Bratthauer GL (1989) Palisaded myofibroblastoma. A benign mesenchymal tumor of lymph node. Am J Surg Pathol 13:341–346
Welsch U, Schwertfirm S, Skirnisson K, Schumacher U (1997) Histological, histochemical, and fine structural observations on the lymph node of the common seal (Phoca vitulina) and the grey seal (Halichoerus grypus). Anat Rec 247:225–242
Willard-Mack CL (2006) Normal structure, function, and histology of lymph nodes. Toxicol Pathol 34:409–424
Wong EY, Morgan L, Smales C, Lang P, Gubby SE, Staddon JM (2000) Vascular endothelial growth factor stimulates dephosphorylation of the catenins p120 and p100 in endothelial cells. Biochem J 346(Pt 1):209–216
Wróbel T, Dziegiel P, Mazur G, Zabel M, Kuliczkowski K, Szuba A (2005) LYVE-1 expression on high endothelial venules (HEVs) of lymph nodes. Lymphology 38:107–110
Wuchter P, Boda-Heggemann J, Straub BK, Grund C, Kuhn C, Krause U, Seckinger A, Peitsch WK, Spring H, Ho AD, Franke WW (2007) Processus and recessus adhaerentes: giant adherens cell junction systems connect and attract human mesenchymal stem cells. Cell Tissue Res 328:499–514
Xiao K, Allison DF, Kottke MD, Summers SA, Sorescu GP, Faundez V, Kowalczyk AP (2003) Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem 278:19199–19208
Xiao K, Garner J, Buckley KM, Vincent PA, Chiasson CM, Dejana E, Faundez V, Kowalczyk AP (2005) p120-Catenin regulates clathrin-dependent endocytosis of VE-cadherin. Mol Biol Cell 16:5141–5151
Yap AS, Crampton MS, Hardin J (2007) Making and breaking contacts: the cellular biology of cadherin regulation. Curr Opin Cell Biol 19:508–514
Yoshida T, Takaya K (1992) The enveloping of intercellular collagenous fibrils by reticular cell processes in postnatal development of rat lymph nodes. Arch Histol Cytol 55:351–359
Zhou X, Stuart A, Dettin LE, Rodriguez G, Hoel B, Gallicano GI (2004) Desmoplakin is required for microvascular tube formation in culture. J Cell Sci 117:3129–3140
Acknowledgements
We thank Renate Baumann, Sabine Koch, and Viktoria Morokina (Marburg) for expert technical help, and Eva Gundel (Heidelberg) for careful typing of the manuscript.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Deutsche Forschungsgemeinschaft (grant MO 345/5–2 to R.M.), the Deutsche Krebshilfe (grant 10–2049-Fr1 to W.W.F.), and the German Ministry for Research and Technology (Program Regenerative Medicine, START-MSC, to W.W.F.).
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
Primary antibodies applied for immunolocalization experiments by using light microscopy of cryostat sections or sections of fixed and paraffin-embedded tissues or for immunoelectron microscopy (DOC 68 kb)
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Moll, R., Sievers, E., Hämmerling, B. et al. Endothelial and virgultar cell formations in the mammalian lymph node sinus: endothelial differentiation morphotypes characterized by a special kind of junction (complexus adhaerens). Cell Tissue Res 335, 109–141 (2009). https://doi.org/10.1007/s00441-008-0700-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-008-0700-y