Abstract
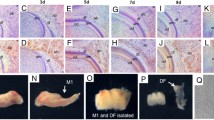
Dental follicle cells in the periodontium are known to have the ability to differentiate into fibroblasts, cementoblasts, and osteoblasts during mouse periodontal development. From embryonic day 14 (E14) to postnatal day 11 (PN11), histological observations showed dramatic alterations in the relative width of the periodontal ligament (PDL)-forming region between the alveolar bone-forming and tooth root-forming area. At PN2, the width of the PDL-forming region showed a minimum, but with a higher expression of NOGGIN and proliferation cell nuclear antigen than the other regions. At PN11, the relative width of the PDL-forming region had expanded. Transplantation of individual regions of the developing tooth germ under the kidney renal capsule showed that dental follicle cells at E14 possessed the potential to develop into mineralized tissue after 3 weeks. These results suggested that the recovery of PDL width at PN11 may have resulted from cell proliferation and molecular interactions between osteogenic factors and their antagonists, such as interactions between bone morphogenetic protein 4 (BMP4) and NOGGIN, simlilar to those observed in suture, limb, and somite formation. To confirm the molecular interaction between BMP4 and NOGGIN, NOGGIN-protein bead implantation onto cultures was employed in vitro. This study thus indicates that harmonious interactions between NOGGIN and BMP in PDL-forming cells, which show higher cell proliferation than neighboring cells, might be important for proper periodontium development.





Similar content being viewed by others
References
Avsian-Kretchmer O, Hsueh AJ (2003) Comparative genomic analysis of the eight-membered-ring cystine-knot-containing bone morphogenetic protein (BMP) antagonists. Mol Endocrinol 18:1–12
Brunet LJ, McMahon JA, McMahon AP, Harland RM (1998) Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science 280:1455–1457
Capdevila J, Johnson RL (1998) Endogenous and ectopic expression of noggin suggests a conserved mechanism for regulation of BMP function during limb and somite patterning. Dev Biol 197:205–217
Cho MI, Garant PR (2000) Development and general structure of the periodontium. Periodontology 24:9–27
D’Souza D, Patel K (1999) Involvement of long- and short-range signalling during early tendon development. Anat Embryol (Berl) 200:367–375
Fujiwara N, Tabata MJ, Endoh M, Ishizeki K, Nawa T (2005) Insulin-like growth factor-I stimulates cell proliferation in the outer layer of Hertwig’s epithelial root sheath and elongation of the tooth root in mouse molars in vitro. Cell Tissue Res 320:69–75
Gazzerro E, Gangji V, Canalis E (1998) Bone morphogenetic proteins induce the expression of noggin, which limits their activity in cultured rat osteoblasts. J Clin Invest 102:2106–2114
Holleville N, Quilhac A, Bontoux M, Monsoro-Burq AH (2003) BMP signals regulate Dlx5 during early avian skull development. Dev Biol 257:177–189
Ito-Kato E, Suzuki N, Maeno M, Takada T, Tanabe N, Takayama T, Ito K, Otsuka K (2004) Effect of carnosine on runt-related transcription factor-2/core binding factor alpha-1 and Sox9 expressions of human periodontal ligament cells. J Periodontal Res 39:199–204
Jin QM, Anusaksathien O, Webb SA, Rutherford RB, Giannobile WV (2003) Gene therapy of bone morphogenetic protein for periodontal tissue engineering. J Periodontol 74:202–213
Jin QM, Zhao M, Economides AN, Somerman MJ, Giannobile WV (2004) Noggin gene delivery inhibits cementoblast-induced mineralization. Connect Tissue Res 45:50–59
Kalajzic I, Kalajzic Z, Hurley MM, Lichtler AC, Rowe DW (2003) Stage specific inhibition of osteoblast lineage differentiation by FGF2 and noggin.J Cell Biochem 88:1168–1176
Kim JY, Cho SW, Lee MJ, Hwang HJ, Lee JM, Lee SI, Muramatsu T, Shimono M, Jung HS (2005) Inhibition of connexin 43 alters Shh and Bmp-2 expression patterns in embryonic mouse tongue. Cell Tissue Res 320:409–415
Lin WL, Chien HH, Cho MI (1999) N-cadherin expression during periodontal ligament cell differentiation in vitro. J Periodontol 70:1039–1045
McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP (1998) Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev 12:1438–1452
Mizuno N, Shiba H, Mouri Y, Xu W, Kudoh S, Kawaguchi H, Kurihara H (2005) Characterization of epithelial cells derived from periodontal ligament by gene expression patterns of bone-related and enamel proteins. Cell Biol Int 29:111–117
Nakamura M, Bringas P Jr, Nanci A, Zeichner-David M, Ashdown B, Slavkin HC (1994) Translocation of enamel proteins from inner enamel epithelia to odontoblasts during mouse tooth development. Anat Rec 238:383–396
Nakamura Y, Nakaya H, Saito N, Wakitani S (2006) Coordinate expression of BMP-2, BMP receptors and Noggin in normal mouse spine. J Clin Neurosci 13:250–256
Pereira RC, Economides AN, Canalis E (2000) Bone morphogenetic proteins induce gremlin, a protein that limits their activity in osteoblasts. Endocrinology 141:4558–4563
Plikus MV, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JG, Snead ML, Chai Y, Chuong CM (2005) Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol Dev 7:440–457
Pourquie O (2001) Skin development: delta laid bare. Curr Biol 10:R425–R428
Saito Y, Yoshizawa T, Takizawa F, Ikegame M, Ishibashi O, Okuda K, Hara K, Ishibashi K, Obinata M, Kawashima H (2002) A cell line with characteristics of the periodontal ligament fibroblasts is negatively regulated for mineralization and Runx2/Cbfa1/Osf2 activity, part of which can be overcome by bone morphogenetic protein-2. J Cell Sci 115:4191–4200
Saito M, Handa K, Kiyono T, Hattori S, Yokoi T, Tsubakimoto T, Harada H, Noguchi T, Toyoda M, Sato S, Teranaka T (2005) Immortalization of cementoblast progenitor cells with Bmi-1 and TERT. J Bone Miner Res 20:50–57
Salingcarnboriboon R, Yoshitake H, Tsuji K, Obinata M, Amagasa T, Nifuji A, Noda M (2003) Establishment of tendon-derived cell lines exhibiting pluripotent mesenchymal stem cell-like property. Exp Cell Res 287:289–300
Schroeder HE (1986) The periodontium. Handbook of microscopic anatomy, vol 5. Springer, Berlin
Schweitzer R, Chyung JH, Murtaugh LC, Brent AE, Rosen V, Olson EN, Lassar A, Tabin CJ (2001) Analysis of the tendon cell fate using Scleraxis, a specific marker for tendons and ligaments. Development 128:3855–3866
Sena K, Morotome Y, Baba O, Terashima T, Takano Y, Ishikawa I (2003) Gene expression of growth and differentiation factors in the developing periodontium of rat molars. J Dent Res 82:166–171
Sodek J, McKee MD (2000) Molecular and cellular biology of alveolar bone. Periodontology 24:99–126
Somerman MJ, Ouyang HJ, Berry JE, Saygin NE, Strayhorn CL, D’Errico JA, Hullinger T, Giannobile WV (1999) Evolution of periodontal regeneration: from the roots’ point of view. J Periodontal Res 34:420–424
Stork S, Stork C, Angerer P, Kothny W, Schmitt P, Wehr U, von Schacky C, Rambeck W (2000) Bone sialoprotein is a specific biochemical marker of bone metabolism in postmenopausal women: a randomized 1-year study. Osteoporosis Int 11:790–796
Tzahor E, Kempf H, Mootoosamy RC, Poon AC, Abzhanov A, Tabin CJ, Dietrich S, Lassar AB (2003) Antagonists of Wnt and BMP signaling promote the formation of vertebrate head muscle. Genes Dev 17:3087–3099
Wolpert L (1998) Pattern formation in epithelial development: the vertebrate limb and feather bud spacing. Philos Trans R Soc Lond Biol 353:871–875
Yoshizawa T, Takizawa F, Iizawa F, Ishibashi O, Kawashima H, Matsuda A, Endo N, Kawashima H (2004) Homeobox protein MSX2 acts as a molecular defense mechanism for preventing ossification in ligament fibroblasts. Mol Cell Biol 24:3460–3472
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by a grant of the Korea Health 21 R&D Project, Ministry of Health and Welfare, Republic of Korea (03-PJ1-PG1-CH08-0001).
Electronic supplementary material
Below each image is a link to a high resolution version.
Supplementary figure 1
PCNA-positive cells were counted in each bone-, PDL-, and tooth-forming regions at PN2. PCNA-positive cells numbered 38.0±2.7/100 μm2 (n=10) in the bone-forming region, 70.2±5.0/100 μm2 (n=10) in the PDL-forming region, and 55.8±4.4/100 μm2 (n=10) in the tooth-forming region. Student’s t test was used. *P<0.01 (GIF: 33.8 kB)
Supplementary figure 2
After 3 days in culture, PCNA-positive cells were examined in the PDL-forming region. In the control (PBS-bead-implanted), PCNA-positive cells numbered 3.4±0.8 in the PDL-forming region. In the NOGGIN-bead-implanted specimens, there were 8.9±1.5 PCNA-positive cells in the PDL-forming region. Student’s t test was used. *P<0.01 (GIF 27.4 kB)
Supplementary figure 4
Alterations of relative width in the tooth-forming region and PDL-forming region were measured after 3 days of culture. The relative width in the tooth-forming region decreases by 85.6 μm (n=30) after NOGGIN treatment. In contrast, the PDL-forming region increases by 113.8 μm (n=30) after NOGGIN bead implantation. These results indicate that NOGGIN inhibits tooth and bone formation. Student’s t test was used. *P<0.01 (GIF 21.5 KB)
Rights and permissions
About this article
Cite this article
Kim, JY., Cho, SW., Hwang, HJ. et al. Evidence for expansion-based temporal BMP4/NOGGIN interactions in specifying periodontium morphogenesis. Cell Tissue Res 330, 123–132 (2007). https://doi.org/10.1007/s00441-007-0434-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0434-2