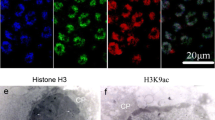
Abstract
The acetylation of core histones has been correlated with the deposition of free histones onto newly replicated DNA, transcriptional activity and the displacement of histones by protamines during spermiogenesis. The aim of the present study was to investigate the immunohistochemical distribution of hyperacetylated H4 during spermatogenesis in Polyodon spathula and to correlate these findings with the pattern of chromatin condensation in spermatids. In immature testis, the Sertoli cells showed more intense immunoreactivity for highly acetylated H4 than that of most primary spermatogonia. The testis of paddlefish at the beginning of spermatogenesis possessed early secondary spermatogonia and late secondary spermatogonia/preleptotene primary spermatocyte with intense nuclear immunoreactivity for highly acetylated H4. In seminiferous tubules in which secondary spermatogonia nuclei were intensely immunostained, Sertoli cell nuclei were unstained. Testes in late spermatogenesis contained tubules in which the immunohistochemical reaction for highly acetylated H4 was positive in the nuclei of preleptotene primary spermatocytes and secondary spermatocytes and in spermatids at the beginning of the elongation process. No immunostaining was found in round spermatids and spermatozoa. During the resting stage, immunostaining was confined to the nuclei of spermatogonia and the cells from the interstitial tissue. Transmission electron microscopy revealed that early spermatids had a round nucleus with filaments of fine chromatin that were dispersed or condensed in clumps. In later stages of maturation, the spermatids had slightly oval nuclei and homogeneous granular chromatin. The chromatin of advanced spermatids was organized into thick fibres. At the end of spermiogenesis, spermatozoan nuclei consisted of homogeneous highly compacted chromatin.








Similar content being viewed by others
References
Adams CR, Kamakaka RT (1999) Chromatin assembly: biochemical identities and genetic redundancy. Curr Opin Genet Dev 9:185–190
Balhorn R, Cosman M, Thornton K, Krishnan VV, Corzett M, Bench G, Kramer C, Lee J, Hud NV, Allen MJ, Prieto M, Meyer-Ilse W, Brown JT, Kirz J, Zhang X, Bradbury EM, Maki G, Braun RE, Breed W (1999) Protamine mediated condensation of DNA in mammalian sperm. In: Gagnon C (ed) The male gamete from basic science to clinical application. Cache River, Vienna, Ill., pp 55–70
Billard R (1990) Spermatogenesis in teleost fish. In: Lamming GE (ed) Marshall’s physiology of reproduction. Churchill Livingstone, Edinburgh, pp 183–212
Bremis W, Findeis EK, Grande L (1997) An overview of Acipenseriformes. Environ Biol Fish 48:28–71
Caron C, Govin J, Rousseaux S, Khochbin S (2005) How to pack the genome for a safe trip. Prog Mol Subcell Biol 38:65–89
Cauty C, Loir M (1995) The interstitial cells of the trout testis (Oncorhynchus mykiss): ultrastructural characterization and changes throughout the reproductive cycle. Tissue Cell 27:383–395
Chaves-Pozo E, Mulero V, Meseguer J, Garcia Ayala A (2005) An overview of cell renewal in the testis throughout the reproductive cycle of a seasonal breeding teleost, the gilthead seabream (Sparus aurata L). Biol Reprod 72:593–601
Chiva M, Lafargue F, Rosenberg E, Kasinsky HE (1992) Protamines, not histones, are the predominant basic proteins in sperm nuclei of solitary ascidian tunicates. J Exp Zool 263:338–349
Christensen ME, Dixon GH (1982) Hyperacetylation of histone H4 correlates with the terminal, transcriptionally inactive stages of spermatogenesis in rainbow trout. Dev Biol 93:404–415
Christensen ME, Rattner JB, Dixon GH (1984) Hyperacetylation of histone H4 promotes chromatin decondensation prior to histone replacement by protamines during spermatogenesis in rainbow trout. Nucleic Acids Res 12:4575–4592
Churikov D, Zalenskaya IA, Zalensky AO (2004) Male germline-specific histones in mouse and man. Cytogenet Genome Res 105:203–214
Doroshov SI, Moberg GP, Van Eenennaam JP (1997) Observations on the reproductive cycle of cultured white sturgeon, Acipenser transmontanus. Environ Biol Fish 48:265–278
Faure AK, Pivot-Pajot C, Kerjean A, Hazzouri M, Pelletier R, Peoc’h M, Sele B, Khochbin S, Rousseaux S (2003) Misregulation of histone acetylation in Sertoli cell-only syndrome and testicular cancer. Mol Hum Reprod 9:757–763
Fenic I, Sonnack V, Failing K, Bergmann M, Steger K (2004) In vivo effects of histone-deacetylase inhibitor trichostatin-A on murine spermatogenesis. J Androl 25:811–818
Ferreira A, Dolder H (2003) Sperm ultrastructure and spermatogenesis in the lizard, Tropidurus itambere. Biocell 27:353–362
Fukumoto M, Zarnescu O (2003) Acrosome differentiation and the acrosome reaction in ascidian spermatozoa: Ascidella aspersa and Ascidia mentula with some implications for tunicate phylogeny. Marine Biol 143:1151–1160
Gimenez-Bonafe P, Ribes E, Sautiere P, Gonzalez A, Kasinsky H, Kouach M, Sautiere PE, Ausio J, Chiva M (2002) Chromatin condensation, cysteine-rich protamine, and establishment of disulphide interprotamine bonds during spermiogenesis of Eledone cirrhosa (Cephalopoda). Eur J Cell Biol 81:341–349
Govin J, Lestrat C, Caron C, Pivot-Pajot C, Rousseaux S, Khochbin S (2006) Histone acetylation-mediated chromatin compaction during mouse spermatogenesis. Ernst Schering Res Found Workshop 57:155–172
Grier HJ (1993) Comparative organization of Sertoli cells including the Sertoli cell barrier. In: Russell LD, Griswold MD (eds) The Sertoli cell. Cache River, Vienna, Ill., pp 703–739
Grimes SR Jr, Henderson N (1984) Hyperacetylation of histone H4 in rat testis spermatids. Exp Cell Res 152:91–97
Grimes SR Jr, Smart PG (1985) Changes in the structural organization of chromatin during spermatogenesis in the rat. Biochim Biophys Acta 824:128–139
Hazzouri M, Pivot-Pajot C, Faure AK, Usson Y, Pelletier R, Sele B, Khochbin S, Rousseaux S (2000) Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histone deacetylases. Eur J Cell Biol 79:950–960
Hebbes TR, Thorne AW, Crane-Robinson C (1988) A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J 7:1395–1402
Jamieson BGM (1999) Spermatozoal phylogeny of the vertebrata. In: Gagnon C (ed) The male gamete: from basic science to clinical applications. Cache River, Vienna, Ill., pp 303–331
Jasencakova Z, Meister A, Walter J, Turner BM, Schubert I (2000) Histone H4 acetylation of euchromatin and heterochromatin is cell cycle dependent and correlated with replication rather than with transcription. Plant Cell 12:2087–2100
Jennings CA, Zigler SJ (2000) Ecology and biology of paddlefish in North America: historical perspectives, management approaches, and research priorities. Rev Fish Biol Fisher 10:167–181
Kaverzneva ED, Rakhmatulina AZ (1972) Isolation and characteristics of the protamine from Acipenser stellatus. Chem Nat Compd 6:111–114
Kennedy BP, Davies PL (1980) Acid-soluble nuclear proteins of the testis during spermatogenesis in the winter flounder. Loss of the high mobility group proteins. J Biol Chem 255:2533–2539
Kennedy BP, Davies PL (1981) Phosphorylation of a group of high molecular weight basic nuclear proteins during spermatogenesis in the winter flounder. J Biol Chem 256:9254–9259
Lahn BT, Tang ZL, Zhou J, Barndt RJ, Parvinen M, Allis CD, Page DC (2002) Previously uncharacterized histone acetyltransferases implicated in mammalian spermatogenesis. Proc Natl Acad Sci USA 99:8707–8712
Lewis JD, Song Y, Jong ME de, Bagha SM, Ausio J (2003) A walk through vertebrate and invertebrate protamines. Chromosoma 111:473–482
Loidl P, Grobner P (1987) Postsynthetic acetylation of histones during the cell cycle: a general function for the displacement of histones during chromatin rearrangements. Nucleic Acids Res 15:8351–8366
Loir M (1989) Trout Sertoli cells and germ cells in primary culture. I. Morphological and ultrastructural study. Gamete Res 24:151–169
Loir M (1999) Spermatogonia of rainbow trout. I. Morphological characterization, mitotic activity, and survival in primary cultures of testicular cells. Mol Reprod Dev 53:422–433
McClusky LM (2005) Stage and season effects on cell cycle and apoptotic activities of germ cells and Sertoli cells during spermatogenesis in the spiny dogfish (Squalus acanthias). Reproduction 129:89–102
Meistrich ML, Trostle-Weige PK, Lin R, Bhatnagar YM, Allis CD (1992) Highly acetylated H4 is associated with histone displacement in rat spermatids. Mol Reprod Dev 31:170–181
Oliva R, Dixon GH (1991) Vertebrate protamine genes and the histone-to-protamine replacement reaction. Prog Nucleic Acid Res Mol Biol 40:25–94
Oliva R, Mezquita C (1982) Histone H4 hyperacetylation and rapid turnover of its acetyl groups in transcriptionally inactive rooster testis spermatids. Nucleic Acids Res 10:8049–8059
Parenti LR, Grier HJ (2004) Evolution and phylogeny of gonad morphology in bony fishes. Integr Comp Biol 44:333–348
Parthun MR, Widom J, Gottschling DE (1996) The major cytoplasmic histone acetyltransferase in yeast: links to chromatin replication and histone metabolism. Cell 87:85–94
Pudney J (1995) Spermatogenesis in nonmammalian vertebrates. Microsc Res Tech 32:459–497
Schulz RW, Menting S, Bogerd J, Franca LR, Vilela DA, Godinho HP (2005) Sertoli cell proliferation in the adult testis—evidence from two fish species belonging to different orders. Biol Reprod 73:891–898
Shaw PJ, Highett MI, Beven AF, Jordan EG (1995) The nucleolar architecture of polymerase I transcription and processing. EMBO J 14:2896–2906
Sonnack V, Failing K, Bergmann M, Steger K (2002) Expression of hyperacetylated histone H4 during normal and impaired human spermatogenesis. Andrologia 34:384–390
Suphamungmee W, Apisawetakan S, Weerachatyanukul W, Wanichanon C, Sretarugsa P, Poomtong T, Sobhon P (2005) Basic nuclear protein pattern and chromatin condensation in the male germ cells of a tropical abalone, Haliotis asinina. Mol Reprod Dev 70:211–221
Wang Q, Yin S, Ai JS, Liang CG, Hou Y, Chen DY, Schatten H, Sun QY (2006) Histone deacetylation is required for orderly meiosis. Cell Cycle 5:766–774
Weisel GF (1943) A histological study of the testis of the sockeye salmon Oncorhynchus nerka. J Morphol 73:207–229
Yulikova EP, Evseenko LK, Bulanov VV, Silaev AB (1974) Protamines fractionation of sturine from Acipenser güldenstädti on carboxymethyl-Sephadex C-25. Chem Nat Compd 8:759–762
Zarnescu O (2005) Ultrastructural study of spermatozoa of the paddlefish, Polyodon spathula. Zygote 13:241–247
Zirkin BR (1975) The ultrastructure of nuclear differentiation during spermiogenesis in the salmon. J Ultrastr Res 50:174–184
Acknowledgements
The author is grateful to Dr. Lotus Mester, Professor of Vertebrate Zoology, for her encouragement to start studies on the paddlefish, and to Dan Vizitiu for kindly providing the fish. The manuscript was much improved by comments and suggestions from two anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zarnescu, O. Immunohistochemical distribution of hyperacetylated histone H4 in testis of paddlefish Polyodon spathula: ultrastructural correlation with chromatin condensation. Cell Tissue Res 328, 401–410 (2007). https://doi.org/10.1007/s00441-006-0373-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0373-3