Abstract
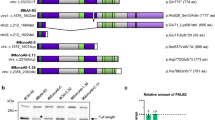
TBX3, the gene mutated in ulnar-mammary syndrome (UMS), is involved in the production of a transcription factor of the T-box family, known to inhibit transcription from the p14ARF (p19ARF in mouse) promoter in fibroblasts and to contribute to cell immortalization. One of the main features of the UMS phenotype is the severe hypoplasia of the breast, associated with haploinsufficiency of the TBX3 gene product. In mice homozygous for the targeted disruption of Tbx3, the mammary glands (MGs) are nearly absent from early stages of embryogenesis, whereas in heterozygous adults, the MGs show reduced ductal branching. All these data strongly suggest a specific role of TBX3 in promoting the growth of mammary epithelial cells (MECs), although direct evidence of this is lacking. Here, we provide data showing the growth-promoting function of Tbx3 in several models of MECs, in association with its ability to repress the ARF promoter. However, no effect of Tbx3 on cell differentiation or apoptosis has been observed. The growth promoting function also entails the down-regulation of p21CIP1/WAF and an increase in cyclin D1 but is independent of p53 and Mdm2 cell-cycle regulatory proteins, as p53-null MECs show similar growth responses associated with the up- or down-regulation of Tbx3. This is the first direct evidence that the level of Tbx3 expression positively controls the proliferation of MECs via pathways alternative to Mdm2-p53.




Similar content being viewed by others
References
Ball R, Friis R, Schoenenberger C, Doppler W, Groner B (1988) Prolactin regulation of b-casein expression and of a cytosolic 120 kDa protein in a cloned mouse mammary epithelial cell. EMBO J 7:2089–2095
Bamshad M, Lin R, Law D, Scott Watkins W, Krakowiak P, Moore M, Franceschini P, Lala R, Holmes L, Gebuhr T, Bruneau B, Schinzel A, Seidman J, Seidman C, Jorde L (1997) Mutations in human TBX3 alter limb, apocrine and genital development in ulnar-mammary syndrome. Nat Genet 16:311–315
Bamshad M, Le T, Watkins W, Dixon M, Kramer B, Roeder AD (1999) The spectrum of mutations in Tbx3. Am J Hum Genet 64:1550–1562
Basson C, Bachinsky D, Lin R, Levi T, Elkins M (1997) Mutations in human TBX5 cause limb and cardiac malformation in Holt-Oram syndrome. Nat Genet 15:30–35
Braybrook C, Doudney K, Marcano A, Arnason A, Bjornsson A, Patton M, Goodfellow P, Moore G, Stainer P (2001) The T-box transcription factor gene TBX22 is mutated in X-linked cleft palate and ankyloglossia. Nat Genet 29:179–183
Brown JP, Wei W, Sedivy JM (1997) Bypass of senescence after disruption of p21CIP1/WAF1 gene in normal diploid human fibroblasts.Science 277:831–834
Brugarolas J, Chandrasekaran C, Gordon JI, Beach D, Jacks T, Hannon GJ (1995) Radiation-induced cell cycle arrest compromised by p21 deficiency. Nature 377:552–557
Brummelkamp T, Kortlever R, Lingbeek M, Trettel F, MacDonald M, Lohuizen M van, Bernards R (2002) TBX3, the gene mutated in ulnar-mammary syndrome, is a negative regulator of p19ARF. J Biol Chem 277:6567–6572
Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, and Seidman JG (2001) A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell 106:709–721
Butz NV, Campbell CE, Gronostajski RM (2004) Differential target gene activation by TBX2 and TBX2VP16: evidence for activation domain-dependent modulation of gene target specificity. Gene 342:67–76
Carlson H, Ota S, Campbell C, Hurlin P (2001) A dominant repression domain in TBX3 mediates transcription repression and cell immortalization: relevance to mutations in TBX3 that cause ulnar-mammary syndrome. Hum Mol Genet 10:2403–2413
Carlson H, Ota S, Song Y, Chen Y, Hurlin P (2002) Tbx3 impinges on the p53 pathway to suppress apoptosis, facilitate cell transformation and block myogenic differentiation. Oncogene 21:3827–3835
Carnero A, Hudson JD, Price CM, Beach DH (2000) p16INK4A and p19ARF act in overlapping pathways in cellular immortalization. Nat Cell Biol 2:148–155
Carreira S, Dexter TJ, Yavuzer U, Easty DJ, Goding CR (1998) Brachyury-related transcription factor Tbx2 and repression of the melanocyte-specific TRP-1 promoter. Mol Cell Biol 18:5099–5108
Chapman D, Garvey N, Hancock S, Alexiou A, Agulnik S (1996) Expression of the T-box family genes, TBX1-TBX5 during early mouse development. Dev Dyn 206:379–390
Coletta RD, Jedlicka P, Gutierrez-Hartmann A, Ford HL (2004) Transcriptional control of the cell cycle in mammary gland development and tumorigenesis. J Mammary Gland Biol Neoplasia 9:39–53
D’Amico M, Wu K, Fu M, Rao M, Albanese C, Russell RG, Lian H, Bregman D, White MA, Pestell RG (2004) The inhibitor of cyclin-dependent kinase 4a/alternative reading frame (INK4a/ARF) locus encoded proteins p16INK4a and p19ARF repress cyclin D1 transcription through distinct cis elements.Cancer Res 64:4122–4130
Daniel CW, Smith GH (1999) The mammary gland: a model for development. J Mammary Gland Biol Neoplasia 4:3–8
Danielson K, Osborn C, Durban E, Butel J, Medina D (1984) Epithelial mouse mammary cell line exhibiting normal morphogenesis in vivo and functional differentiation in vitro. Proc Natl Acad Sci USA 81:3756–3760
Datta A, Nag A, Raychaudhuri P (2002) Differential regulation of E2F1, DP1, and the E2F1/DP1 complex by ARF. Mol Cell Biol 22:8398–8408
Davenport T, Jerome-Majewska L, Papaioannou V (2003) Mammary gland, limb and yolk sac defects in mice lacking TBX3, the gene mutated in human ulnar mammary syndrome. Development 130:2263–2273
Docquier F, Farrar D, D’Arcy V, Chernukhin I, Robinson AF, Loukinov D, Vatolin S, Pack S, Mackay A, Harris RA, Dorricott H, O’Hare MJ, Lobanenkov V, Klenova E (2005) Heightened expression of CTCF in breast cancer cells is associated with resistance to apoptosis. Cancer Res 15:5112–5122
Dulbecco R, Okada S (1980) Differentiation and morphogenesis of mammary cells in vitro. Proc R Soc Lond [Biol] 208:399–408
Dulbecco R, Bologna M, Unger M (1979) Differentiation of a rat mammary cell line in vitro. Proc Natl Acad Sci USA 76:1256–1260
Dulbecco R, Bologna M, Unger M (1980) Control of differentiation of a mammary cell line by lipids. Proc Natl Acad Sci USA 77:1551–1555
Gartel AL, Tyner AL (2002) The role of the cyclin-dependent kinase inhibitor p21 in apoptosis. Mol Cancer Ther 1:639–649
Govoni KE, Lee SK, Chadwick RB, Yu H, Kasukawa Y, Baylink DJ, Mohan S (2006) Whole genome microarray analysis of growth hormone induced gene expression in bone: T-box3, a novel transcription factor, regulates osteoblast proliferation. Am J Physiol Endocrinol Metab 291:E128–E136
Groner B (2002) Transcription factor regulation in mammary epithelial cells. Domest Anim Endocrinol 23:25–32
He ML, Wen L, Campbell CE, Wu JY, Rao Y (1999) Transcription repression by Xenopus ET and its human ortholog TBX3, a gene involved in ulnar-mammary syndrome. Proc Natl Acad Sci USA 96:10212–10217
Hennighausen L, Robinson G (1998) Think globally, act locally: the making of a mouse mammary gland. Genes Dev 12:449–455
Ito A, Asamoto M, Hokaiwado N, Takahashi S, Shirai T (2005) Tbx3 expression is related to apoptosis and cell proliferation in rat bladder both hyperplastic epithelial cells and carcinoma cells. Cancer Lett 219:105–112
Jacobs JJL, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof PM, Welsem T van, Vijver MJ van de, Koh EY, Daley GQ, Lohuizen M van (2000) Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19ARF) and is amplified in a subset of human breast cancers. Nat Genet 26:291–299
Jerome L, Papaioannou V (2001) DiGeorge syndrome phenotype in mice mutant for the T-box gene TBX1. Nat Genet 27:286–291
Jerome-Majewska L, Jenkins G, Ernstoff E, Zindy F, Sherr C, Papaioannou V (2005) Tbx3, the ulnar-mammary syndrome gene, and Tbx2 interact in mammary gland development through a p19ARF/p53-independent pathway. Dev Dyn 234:922–933
Jerry DJ, Kuperwasser C, Downing SR, Pinkas J, He C, Dickinson ES, Marconi S, Naber SP (1998) Delayed involution of the mammary epithelium in BALB/c-p53 null mice. Oncogene 17:2305–2312
Kashuba E, Mattsson K, Klein G, Szekely L (2003) p14ARF induces the relocation of HDM2 and p53 to extranucleolar sites that are targeted by PML bodies and proteasomes. Mol Cancer 5:2–18
Koopman G, Reutelingsperger CPM, Kuijten GAM, Keelmen RMJ, Pals ST, Oers MHJ van (1994) Annexin V for flow cytometric detection of phosphatidylserine expression on B cells undergoing apoptosis. Blood 84:1415–1420
Korgaonkar C, Zhao L, Modestou M, Quelle DE (2002) ARF function does not require p53 stabilization or Mdm2 Relocalization. Mol Cell Biol 22:196–206
Li M, Newbury-Ecob R, Terret J, Wilson D, Curtis A (1997) Holt-Oram syndrome is caused by mutations in TBX5, a member of the brachyury gene family. Nat Genet 15:21–29
Lindsay E, Vitelli F, Su H, Morishima M (2001) TBX1 haploinsufficiency in the DiGeorge syndrome region causes aortic arch defects in mice. Nature 410:97–101
Lingbeek M, Jacobs J, Lohuizen M van (2002) The T-box repressors TBX2 and TBX3 specifically regulate the tumor suppressor gene p14ARF via a variant T-site. J Biol Chem 277:26120–26127
Marcano ACB, Doudney K, Braybrook C, Squires R, Patton MA, Lees MM, Richieri-Costa A, Lidral AC, Murray JC, Moore GE, Stanier P (2004) TBX22 mutations are a frequent cause of cleft palate. J Med Genet 41:68–74
Medina D, Kittrell FS (2003) p53 function is required for hormone-mediated protection of mouse mammary tumorigenesis. Cancer Res 63:6140–6143
Meneghini V, Odent S, Platonova N, Egeo A, Merlo GR (2006) Novel TBX3 mutation data in families with ulnar-mammary syndrome indicate a genotype-phenotype relationship: mutations that do not disrupt the T-domain are associated with less severe limb defects. Eur J Med Genet 49:151–158
Merlo GR, Venesio T, Taverna D, Marte B, Callahan R, Hynes N (1994) Growth suppression of normal mammary epithelial cells by wild-type p53. Oncogene 9:443–453
Merlo G, Fiore L, Basolo F, Duboc L, Hynes N (1995) p53-dependent and p53-independent apoptosis of mammary epithelial cells reveals a role for EGF and insulin as survival factors. J Cell Biol 128:1185–1196
Merlo G, Graus-Porta D, Cella N, Marte B, Taverna D, Hynes N (1996) Growth, differentiation and survival of HC11 mammary epithelial cells: diverse effects of receptor tyrosine kinase-activating growth factors. Eur J Cell Biol 70:97–105
Merscher S, Funke B, Epstein J, Heyer J, Puech A, Lu M (2001) TBX1 is responsible for cardiovascular defects in velo-cardio-facial/DiGeorge syndrome. Cell 104:619–629
Momand J, Zambetti GP (1997) Mdm-2: “big brother” of p53. J Cell Biochem 64:343–352
Morgenstern JP, Land H (1990) Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res 18:3587–3596
Moskowitz IP, Pizard A, Patel VV, Bruneau BG, Kim JB, Kupershmidt S, Roden D, Berul CI, Seidman CE, Seidman JG (2004) The T-box transcription factor Tbx5 is required for the patterning and maturation of the murine cardiac conduction system. Development 131:4107–4116
Naiche L, Papaioannou V (2003) Loss of TBX4 blocks hindlimb development and affects vascularization and fusion of the allantois. Development 130:2681–2693
Normand G, Hemmati PG, Verdoodt B, Haefen C von, Wendt J, Guner D, May E, Dorken B, Daniel PT (2005) p14ARF induces G2 cell cycle arrest in p53- and p21-deficient cells by down-regulating p34cdc2 kinase activity. J Biol Chem 280:7118–7130
Packham E, Brook J (2003) T-box genes in human disorders. Hum Mol Genet 12:R37–R44
Papaioannou V, Silver L (1998) The T-box gene family. BioEssays 20:9–19
Paxton C, Zhao H, Chin Y, Langner K, Reecy J (2002) Murine TBX2 contains domains that activate and repress gene transcription. Gene 283:117–124
Prince S, Carreira S, Vance KW, Abrahams A, Goding CR (2004) TBX2 directly represses the expression of the p21WAF1 cyclin-dependent kinase inhibitor. Cancer Res 64:1669–1674
Resnitzky D, Reed S (1995) Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol 15:3463–3469
Rowley M, Grothey E, Couch FJ (2004) The role of TBX2 and TBX3 in mammary development and tumorigenesis. J Mammary Gland Biol Neoplasia 9:109–118
Sherr CJ (1998) Tumor surveillance via the ARF-p53 pathway. Genes Dev 12:2984–2991
Smith J (1999) T-box genes: what they do and how they do it. Trends Genet 15:154–158
Steinman HA, Burstein E, Lengner C, Gosselin J, Pihan G, Duckett CS, Jones SN (2004) An alternative splice form of mdm2 induces p53-independent cell growth and tumorigenesis. J Biol Chem 279:4877–4886
Sugimoto M, Kuo ML, Roussel MF, Sherr CJ (2003) Nucleolar ARF tumor suppressor inhibits ribosomal RNA processing. Mol Cell 11:415–424
Tada M, Smith J (2001) T-targets: clues to understanding the functions of T-box proteins. Dev Growth Differ 43:1–11
Veltmaat J, Maileux A, Thiery J, Bellusci S (2003) Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation 71:1–17
Visvader JE, Lindeman GJ (2003) Transcriptional regulators in mammary gland development and cancer. Int J Biochem Cell Biol 35:1034–1051
Wang Y, Blandino G, Givol D (1999) Induced p21waf expression in H1299 cell line promotes cell senescence and protects against cytotoxic effect of radiation and doxorubicin. Oncogene 18:2643–2649
Weber JD, Taylor LJ, Roussel MF, Sherr CJ, Bar-Sagi D (1999) Nucleolar ARF sequesters Mdm2 and activates p53. Nat Cell Biol 1:20–26
Weber JD, Jeffers JR, Rehg JE, Randle DH, Lozano G, Roussel MF, Sherr CJ, Zambetti GP (2000) p53-Independent functions of the p19 (ARF) tumor suppressor. Genes Dev 14:2358–2365
Weinstat-Saslow D, Merino M, Manrow R, Lawrence J, Bluth R, Wittenbel K, Simpson J, Page D, Steeg P (1995) Overexpression of cyclin D mRNA distinguishes invasive and in situ breast carcinomas from non-malignant lesions. Nat Med 1:1257–1259
Wilson V, Conlon FL (2002) The T-box family. Genome Biol 3:3008.1–3008.7
Yarbrough WG, Bessho M, Zanation A, Bisi JE, Xiong Y (2002) Human tumor suppressor ARF impedes S-phase progression independent of p53. Cancer Res 62:1171–1177
Yi Y, Shepard A, Kittrell F, Mulac-Jericevic B, Medina D, Said TK (2004) p19ARF determines the balance between normal cell proliferation rate and apoptosis during mammary gland development. Mol Biol Cell 15:2302–2311
Zucchi I, Montagna C, Susani L, Vezzoni P, Dulbecco R (1998) The rat gene homologous to the human gene 9–27 is involved in the development of the mammary gland. Proc Natl Acad Sci USA 95:1079–1084
Zucchi I, Montagna C, Susani L, Montesano R, Affer M, Zanotti S, Redolfi E, Vezzoni P, Dulbecco R (1999) Genetic dissection of dome formation in a mammary cell line: identification of two genes with opposing action. Proc Natl Acad Sci USA 98:13766–13770
Zucchi I, Bini L, Albani D, Valaperta R, Liberatori S, Raggiaschi R, Montagna C, Susani L, Barbieri O, Pallini V, Vezzoni P, Dulbecco R (2002) Dome formation in cell cultures as expression of an early stage of lactogenic differentiation of the mammary gland. Proc Natl Acad Sci USA 99:8660–8665
Acknowledgements
We are grateful to Dr. M. Lingbeek (The Netherlands Cancer Institute, Amsterdam) and Drs. P. Hurlin and S. Ota (Shriners Hospitals, Portland, USA) for the generous gift of plasmids and to Dr. N. Hynes (Friedrich Miescher Institute, Basel, Switzerland) and Dr. D. Medina (Baylor College of Medicine, Houston, USA) for the HC11 and NulliB cells. We thank Dr. S. Mantero (Dulbecco Telethon Institute, Milano), Dr. E. Erba (Mario Negri Institute, Milano) and Dr. S. Carrabino (Cystic Fibrosis Institute, Milano) for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
G.R.M. is a recipient of a Career Award from Fondazione Telethon, Italy (S99003) and is supported by the Italian Ministry of Foreign Affairs (10-RB18), Fondazione Cariplo (S00083FCRA), Fondazione SanPaolo (99003CSPC) and Istituto Superiore di Sanità. N.P. is in receipt of a NATO-CNR Advanced Fellowship (N215-36S). I.Z. is supported by AIRC Italy (N. 115), Fondazione Cariplo (2003/1656), Italy-USA Project on Cancer Pharmacogenomics (N. 527/B-B7) and Italian Ministry of University and Research-FIRB Internazionale (RBIN04CBSM_000). The work of I.Z. and G.R.M. is also financed by a grant from Fondazione Telethon (GGP04247).
Rights and permissions
About this article
Cite this article
Platonova, N., Scotti, M., Babich, P. et al. TBX3, the gene mutated in ulnar-mammary syndrome, promotes growth of mammary epithelial cells via repression of p19ARF, independently of p53. Cell Tissue Res 328, 301–316 (2007). https://doi.org/10.1007/s00441-006-0364-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0364-4