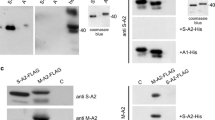
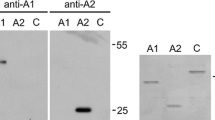
Abstract
Raver1, a ubiquitously expressed protein, was originally identified as a ligand for metavinculin, the muscle-specific isoform of the microfilament-associated protein vinculin. The protein resides primarily in the nucleus, where it colocalises and may interact with polypyrimidine-tract-binding protein, which is involved in alternative splicing processes. During skeletal muscle differentiation, raver1 translocates to the cytoplasm and eventually targets the Z-line of sarcomeres. Here, it colocalises with metavinculin, vinculin and alpha-actinin, all of which have biochemically been identified as raver1 ligands. To obtain more information about the potential role of raver1 in muscle structure and function, we have investigated its distribution and fine localisation in mouse striated and smooth muscle, by using three monoclonal antibodies that recognise epitopes in different regions of the raver1 protein. Our immunofluorescence and immunoelectron-microscopic results indicate that the cytoplasmic accumulation of raver1 is not confined to skeletal muscle but also occurs in heart and smooth muscle. Unlike vinculin and metavinculin, cytoplasmic raver1 is not restricted to costameres but additionally represents an integral part of the sarcomere. In isolated myofibrils and in ultrathin sections of skeletal muscle, raver1 has been found concentrated at the I-Z-I band. A minor fraction of raver1 is present in the nuclei of all three types of muscle. These data indicate that, during muscle differentiation, raver1 might link gene expression with structural functions of the contractile machinery of muscle.






Similar content being viewed by others
References
Bang ML, Mudry RE, McElhinny AS, Trombitas K, Geach AJ, Yamasaki R, Sorimachi H, Granzier H, Gregorio CC, Labeit S (2001) Myopalladin, a novel 145-kilodalton sarcomeric protein with multiple roles in Z-disc and I-band protein assemblies. J Cell Biol 153:413–427
Clark KA, McElhinny AS, Beckerle MC, Gregorio CC (2002) Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol 18:637–706
Frank R, Overwin H (1996) SPOT synthesis. Epitope analysis with arrays of synthetic peptides prepared on cellulose membranes. Methods Mol Biol 66:149–169
Ghetti A, Pinol-Roma S, Michael WM, Morandi C, Dreyfuss G (1992) hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res 20:3671–3678
Gromak N, Rideau A, Southby J, Scadden AD, Gooding C, Huttelmaier S, Singer RH, Smith CW (2003) The PTB interacting protein raver1 regulates alpha-tropomyosin alternative splicing. EMBO J 22:6356–6364
Huttelmaier S, Illenberger S, Grosheva I, Rudiger M, Singer RH, Jockusch BM (2001) Raver1, a dual compartment protein, is a ligand for PTB/hnRNPI and microfilament attachment proteins. J Cell Biol 155:775–786
Janmey PA (1998) The cytoskeleton and cell signaling: component localization and mechanical coupling. Physiol Rev 78:763–781
Jeyaseelan R, Poizat C, Baker RK, Abdishoo S, Isterabadi LB, Lyons GE, Kedes L (1997) A novel cardiac-restricted target for doxorubicin. CARP, a nuclear modulator of gene expression in cardiac progenitor cells and cardiomyocytes. J Biol Chem 272:22800–22808
Jockusch H, Voigt S (2003) Migration of adult myogenic precursor cells as revealed by GFP/nLacZ labelling of mouse transplantation chimeras. J Cell Sci 116:1611–1616
Jockusch BM, Huttelmaier S, Illenberger S (2003) From the nucleus toward the cell periphery: a guided tour for mRNAs. News Physiol Sci 18:7–11
Kleinhenz B, Fabienke M, Swiniarski S, Wittenmayer N, Kirsch J, Jockusch BM, Arnold HH, Illenberger S (2005) Raver2, a new member of the hnRNP family. FEBS Lett 579:4254–4258
Koteliansky VE, Gneushev GN (1983) Vinculin localization in cardiac muscle. FEBS Lett 159:158–160
Lange S, Auerbach D, McLoughlin P, Perriard, E, Schafer BW, Perriard JC, Ehler E (2002) Subcellular targeting of metabolic enzymes to titin in heart muscle may be mediated by DRAL/FHL-2. J Cell Sci 115:4925–4936
Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, et al (2005) The kinase domain of titin controls muscle gene expression and protein turnover. Science 308:1599–1603
Lange S, Ehler E, Gautel M (2006) From A to Z and back? Multicompartment proteins in the sarcomere. Trends Cell Biol 16:11–18
Littlefield R, Almenar-Queralt A, Fowler VM (2001) Actin dynamics at pointed ends regulates thin filament length in striated muscle. Nat Cell Biol 3:544–551
Martin AF (1981) Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem 256:964–968
Mayboroda O, Schluter K, Jockusch BM (1997) Differential colocalization of profilin with microfilaments in PtK2 cells. Cell Motil Cytoskeleton 37:166–177
McKenna NM, Johnson CS, Wang YL (1986) Formation and alignment of Z lines in living chick myotubes microinjected with rhodamine-labeled alpha-actinin. J Cell Biol 103:2163–2171
Michele DE, Albayya FP, Metzger JM (1999) Thin filament protein dynamics in fully differentiated adult cardiac myocytes: toward a model of sarcomere maintenance. J Cell Biol 145:1483–1495
Miller MK, Bang ML, Witt CC, Labeit D, Trombitas C, Watanabe K, Granzier H, McElhinny AS, Gregorio CC, Labeit S (2003) The muscle ankyrin repeat proteins: CARP, ankrd2/Arpp and DARP as a family of titin filament-based stress response molecules. J Mol Biol 333:951–964
Morris EJ, Fulton AB (1994) Rearrangement of mRNAs for costamere proteins during costamere development in cultured skeletal muscle from chicken. J Cell Sci 107:377–386
Pardo JV, Siliciano JD, Craig SW (1983) A vinculin-containing cortical lattice in skeletal muscle: transverse lattice elements (“costameres”) mark sites of attachment between myofibrils and sarcolemma. Proc Natl Acad Sci USA 80:1008–1012
Russell B, Motlagh D, Ashley WW (2000) Form follows function: how muscle shape is regulated by work. J Appl Physiol 88:1127–1132
Ruwhof C, Laarse A van der (2000) Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res 47:23–37
Sanchez-Carbayo M, Schwarz K, Charytonowicz E, Cordon-Cardo C, Mundel P (2003) Tumor suppressor role for myopodin in bladder cancer: loss of nuclear expression of myopodin is cell-cycle dependent and predicts clinical outcome. Oncogene 22:5298–5305
Slot JW, Geuze HJ (1985) A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol 38:87–93
Spellman R, Rideau A, Matlin A, Gooding C, Robinson F, McGlincy N, Grellscheid SN, Southby J, Wollerton M, Smith CW (2005) Regulation of alternative splicing by PTB and associated factors. Biochem Soc Trans 33:457–460
Tokuyasu KT, Dutton AH, Geiger B, Singer SJ (1981) Ultrastructure of chicken cardiac muscle as studied by double immunolabeling in electron microscopy. Proc Natl Acad Sci USA 78:7619–7623
Valcarcel J, Gebauer F (1997) Post-transcriptional regulation: the dawn of PTB. Curr Biol 7:R705–708
Wang J, Shaner N, Mittal B, Zhou Q, Chen J, Sanger JM, Sanger JW (2005) Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil Cytoskeleton 61:34–48
Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P (2001) Differentiation- and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin-bundling protein. J Cell Biol 155:393–404
Witt S, Zieseniss A, Fock U, Jockusch BM, Illenberger S (2004) Comparative biochemical analysis suggests that vinculin and metavinculin cooperate in muscular adhesion sites. J Biol Chem 279:31533–31543
Young P, Ehler E, Gautel M (2001) Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly. J Cell Biol 154:123–136
Zak R, Martin AF, Prior G, Rabinowitz M (1977) Comparison of turnover of several myofibrillar proteins and critical evaluation of double isotope method. J Biol Chem 252:3430–3435
Acknowledgments
We thank R. Frank for providing us with the raver1 spot-synthesized peptides (Helmholtz Centre for Infection Research, Braunschweig) and Tania Messerschmidt (TU Braunschweig) for technical assistance. We gratefully acknowledge permission from B. Kleinhenz and H.H. Arnold (TU Braunschweig) to quote their unpublished results and the assistance of V. Oliveri and U. Sauder (Biozentrum, Basel) with sample preparation for electron microscopy.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by grants from the Swiss National Science Foundation and the M.E. Müller Foundation (to C.A.S.) and the Deutsche Forschungsgemeinschaft (to S.I. and B.M.J.) and from the Fonds der Chemischen Industrie (to B.M.J.). A.Z. was the recipient of a G. Lichtenberg fellowship, within an International Graduate College funded by the State of Lower Saxony, Germany.
Rights and permissions
About this article
Cite this article
Zieseniss, A., Schroeder, U., Buchmeier, S. et al. Raver1 is an integral component of muscle contractile elements. Cell Tissue Res 327, 583–594 (2007). https://doi.org/10.1007/s00441-006-0322-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-006-0322-1