Abstract
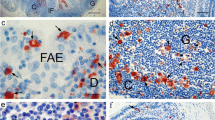
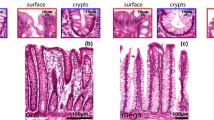
Membranous (M) cells of the follicle-associated epithelium (FAE) are believed to sample antigens from the gut lumen. However, the origin, differentiation mechanism, and cell death of M cells are still a matter of controversy. Therefore, we investigated the process of M cell differentiation and determined their fate in the intestine of three-way crossbred female pigs. We used anti-cytokeratin 18 and anti-PCNA antibodies to distinguish M cells and proliferative cells and performed immunohistochemistry, enzyme histochemistry, and scanning electron microscopy on fresh ileal Peyer’s patches. Cell migration and apoptotic cells were detected by BrdU labeling and the TUNEL method, respectively. The turnover of the FAE was similar to that of the villi. M cells were mostly observed from the FAE crypt to the FAE periphery, but not in the FAE apex. As proliferative M cells (cytokeratin 18+/PCNA+ cells) have previously been detected in the FAE crypt, porcine M cells may be directly derived from intestinal epithelial stem cells and committed as a distinct cell lineage in the crypts. M cells from the FAE periphery were unstained or only weakly stained for alkaline phosphatase, whereas cytokeratin 18+/alkaline phosphatase+ cells lying near to the FAE apex showed a columnar shape similar to that of adjacent enterocytes. These data suggest that the committed M cells differentiate to mature M cells by contact with lymphocytes at the FAE periphery, and that they trans-differentiate to enterocytes and are finally excluded near the FAE apex.





Similar content being viewed by others
References
Bockman DE (1983) Functional histology of appendix. Arch Histol Jpn 46:271–292
Borghesi C, Taussig MJ, Nicoletti C (1999) Rapid appearance of M cells after microbial challenge is restricted at the periphery of the follicle-associated epithelium of Peyer’s patch. Lab Invest 79:1393–1401
Bye WA, Allan CH, Trier JS (1984) Structure distribution and origin of M cells in Peyer’s patches of mouse ileum. Gastroenterology 86:789–801
Carroll KM, Wong TT, Drabik DL, Chang EB (1988) Differentiation of rat small intestinal epithelial cells by extracellular matrix. Am J Physiol 254:G355–G360
Cheng H, Leblond CP (1974a) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat 141:461–479
Cheng H, Leblond CP (1974b) Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. III. Entero-endocrine cells. Am J Anat 141:503–519
Cheng H, Leblond CP (1974c) Origin differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian theory of the origin of the four epithelial cell types. Am J Anat 141:537–561
Clark MA, Jepson MA, Simmons NL, Booth TA, Hirst BH (1993) Differential expression of lectin-binding sites defines mouse intestinal M-cells. J Histochem Cytochem 41:1679–1687
Clark MA, Hirst BH, Jepson MA (1998) M-cell surface beta1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer’s patch M cells. Infect Immun 66:1237–1243
Debard N, Sierro F, Browning J, Kraehenbuhl JP (2001) Effect of mature lymphocytes and lymphotoxin on the development of the follicle-associated epithelium and M cells in mouse Peyer’s patches. Gastroenterology 120:1173–1182
Domon-Dell C, Wang Q, Kim S, Kedinger M, Evers BM, Freund JN (2002) Stimulation of the intestinal Cdx2 homeobox gene by butyrate in colon cancer cells. Gut 50:525–529
Engle MJ, Goetz GS, Alpers DH (1998) Caco-2 cells express a combination of colonocyte and enterocyte phenotypes. J Cell Physiol 174:362–369
Fotopoulos G, Harari A, Michetti P, Trono D, Pantaleo G, Kraehenbuhl JP (2002) Transepithelial transport of HIV-1 by M cells is receptor-mediated. Proc Natl Acad Sci USA 99:9410–9414
Grasset E, Pinto M, Dussaulx E, Zweibaum A, Desjeux JF (1984) Epithelial properties of human colonic carcinoma cell line Caco-2: electrical parameters. Am J Physiol 247:C260–C267
Grasset E, Bernabeu J, Pinto M (1985) Epithelial properties of human colonic carcinoma cell line Caco-2: effect of secretagogues. Am J Physiol 248:C410–C418
Gebert A, Posselt W (1997) Glycoconjugate expression defines the origin and differentiation pathway of intestinal M-cells. J Histochem Cytochem 45:1341–1350
Gebert A, Hach G, Bartels H (1992) Co-localization of vimentin and cytokeratins in M-cells of rabbit gut-associated lymphoid tissue (GALT). Cell Tissue Res 269:331–340
Gebert A, Rothkotter HJ, Pabst R (1994) Cytokeratin 18 is an M-cell marker in porcine Peyer’s patches. Cell Tissue Res 276:213–221
Gebert A, Fassbender S, Werner K, Weissferdt A (1999) The development of M cells in Peyer’s patches is restricted to specialized dome-associated crypts. Am J Pathol 154:1573–1582
Gebert A, Steinmetz I, Fassbender S, Wendlandt KH (2004) Antigen transport into Peyer’s patches: increased uptake by constant numbers of M cells. Am J Pathol 164:65–72
Giannasca PJ, Giannasca KT, Falk P, Gordon JI, Neutra MR (1994) Regional differences in glycoconjugates of intestinal M cells in mice: potential targets for mucosal vaccines. Am J Physiol 267:G1108–G1121
Golovkina TV, Shlomchik M, Hannum L, Chervonsky A (1999) Organogenic role of B lymphocytes in mucosal immunity. Science 286:1965–1968
Groos S, Reale E, Luciano L (2003) General suitability of techniques for in situ detection of apoptosis in small intestinal epithelium. Anat Rec [A] Discov Mol Cell Evol Biol 272:503–513
Gullberg E, Leonard M, Karlsson J, Hopkins AM, Brayden D, Baird AW, Artursson P (2000) Expression of specific markers and particle transport in a new human intestinal M-cell model. Biochem Biophys Res Commun 279:808–813
Hathaway LJ, Kraehenbuhl JP (2000) The role of M cells in mucosal immunity. Cell Mol Life Sci 57:323–332
Helander A, Silvey KJ, Mantis NJ, Hutchings AB, Chandran K, Lucas WT, Nibert ML, Neutra MR (2003) The viral sigma1 protein and glycoconjugates containing alpha2–3–linked sialic acid are involved in type 1 reovirus adherence to M cell apical surfaces. J Virol 77:7964–7977
Jepson MA, Mason CM, Bennett MK, Simmons NL, Hirst BH (1992) Co-expression of vimentin and cytokeratins in M cells of rabbit intestinal lymphoid follicle-associated epithelium. Histochem J 24:33–39
Kedinger M, Lefebvre O, Duluc I, Freund JN, Simon-Assmann P (1998) Cellular and molecular partners involved in gut morphogenesis and differentiation. Philos Trans R Soc Lond Biol 353:847–856
Kerneis S, Bogdanova A, Kraehenbuhl JP, Pringault E (1997) Conversion by Peyer’s patch lymphocytes of human enterocytes into M cells that transport bacteria. Science 277:949–952
Kerneis S, Caliot E, Stubbe H, Bogdanova A, Kraehenbuhl J, Pringault E (2000) Molecular studies of the intestinal mucosal barrier physiopathology using cocultures of epithelial and immune cells: a technical update. Microbes Infect 2:1119–1124
Kraehenbuhl JP, Neutra MR (2000) Epithelial M cells: differentiation and function. Annu Rev Cell Dev Biol 16:301–332
Kucharzik T, Lugering N, Schmid KW, Schmidt MA, Stoll R, Domschke W (1998) Human intestinal M cells exhibit enterocyte-like intermediate filaments. Gut 42:54–62
Kucharzik T, Lugering N, Rautenberg K, Lugering A, Schmidt MA, Stoll R, Domschke W (2000) Role of M cells in intestinal barrier function. Ann N Y Acad Sci 915:171–183
Lelouard H, Sahuquet A, Reggio H, Montcourrier P (2001) Rabbit M cells and dome enterocytes are distinct cell lineages. J Cell Sci 114:2077–2083
Mariadason JM, Rickard KL, Barkla DH, Augenlicht LH, Gibson PR (2000) Divergent phenotypic patterns and commitment to apoptosis of Caco-2 cells during spontaneous and butyrate-induced differentiation. J Cell Physiol 183:347–354
Mariadason JM, Velcich A, Wilson AJ, Augenlicht LH, Gibson PR (2001) Resistance to butyrate-induced cell differentiation and apoptosis during spontaneous Caco-2 cell differentiation. Gastroenterology 120:889–899
Marshman E, Booth C, Potten CS (2002) The intestinal epithelial stem cell. Bioessays 24:91–98
Meyerholz DK, Stabel TJ, Ackermann MR, Carlson SA, Jones BD, Pohlenz J (2002) Early epithelial invasion by Salmonella enterica serovar Typhimurium DT104 in the swine ileum. Vet Pathol 39:712–720
Neutra MR (1998) Current concepts in mucosal immunity. V. Role of M cells in transepithelial transport of antigens and pathogens to the mucosal immune system. Am J Physiol 274:G785–G791
Neutra MR (1999) M cells in antigen sampling in mucosal tissues. Curr Top Microbiol Immunol 236:17–32
Neutra MR, Phillips TL, Mayer EL, Fishkind DJ (1987) Transport of membrane-bound macromolecules by M cells in follicle-associated epithelium of rabbit Peyer’s patch. Cell Tissue Res 247:537–546
Niedergang F, Kraehenbuhl JP (2000) Much ado about M cells. Trends Cell Biol 10:137–141
Owen RL (1977) Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer’s patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology 72:440–451
Owen RL, Bhalla DK (1983) Cytochemical analysis of alkaline phosphatase and esterase activities and of lectin-binding and anionic sites in rat and mouse Peyer’s patch M cells. Am J Anat 168:199–212
Owen RL, Jones AL (1974) Epithelial cell specialization within human Peyer’s patches: an ultrastructural study of intestinal lymphoid follicles. Gastroenterology 66:189–203
Porta C, James PS, Phillips AD, Savidge TC, Smith MW, Cremaschi D (1992) Confocal analysis of fluorescent bead uptake by mouse Peyer’s patch follicle-associated M cells. Exp Physiol 77:929–932
Potten CS (1998) Stem cells in gastrointestinal epithelium: numbers, characteristics and death. Philos Trans R Soc Lond Biol 353:821–830
Rautenberg K, Cichon C, Heyer G, Demel M, Schmidt MA (1996) Immunocytochemical characterization of the follicle-associated epithelium of Peyer’s patches: anti-cytokeratin 8 antibody (clone 4.1.18) as a molecular marker for rat M cells. Eur J Cell Biol 71:363–370
Regoli M, Bertelli E, Borghesi C, Nicoletti C (1995) Three-dimensional (3D-) reconstruction of M cells in rabbit Peyer’s patches: definition of the intraepithelial compartment of the follicle-associated epithelium. Anat Rec 243:19–26
Sansonetti PJ, Arondel J, Cantey JR, Prevost MC, Huerre M (1996) Infection of rabbit Peyer’s patches by Shigella flexneri: effect of adhesive or invasive bacterial phenotypes on follicle-associated epithelium. Infect Immun 64:2752–2764
Savidge TC (1996) The life and times of an intestinal M cell. Trends Microbiol 4:301–306
Savidge TC, Smith MW, James PS, Aldred P (1991) Salmonella-induced M-cell formation in germ-free mouse Peyer’s patch tissue. Am J Pathol 139:177–184
Sicinski P, Rowinski J, Warchol JB, Bem W (1986) Morphometric evidence against lymphocyte-induced differentiation of M cells from absorptive cells in mouse Peyer’s patches. Gastroenterology 90:609–616
Sierro F, Pringault E, Assman PS, Kraehenbuhl JP, Debard N (2000) Transient expression of M-cell phenotype by enterocyte-like cells of the follicle-associated epithelium of mouse Peyer’s patches. Gastroenterology 119:734–743
Westcarr S, Farshori P, Wyche J, Anderson WA (1999) Apoptosis and differentiation in the crypt-villus unit of the rat small intestine. J Submicrosc Cytol Pathol 31:15–30
Author information
Authors and Affiliations
Corresponding author
Additional information
This investigation was supported by a Grant-in-Aid for Scientific Research (16658107) from the Ministry of Education, Culture, Sports, Science and Technology, by two grants (Prion Project and Secure and Healthy Livestock Farming Project) from the Ministry of Agriculture, Forestry and Fisheries, and by a grant from the Naito Foundation.
Rights and permissions
About this article
Cite this article
Miyazawa, K., Aso, H., Kanaya, T. et al. Apoptotic process of porcine intestinal M cells. Cell Tissue Res 323, 425–432 (2006). https://doi.org/10.1007/s00441-005-0086-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-005-0086-z