Abstract
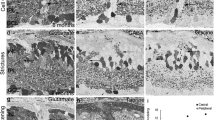
DBA/2J (D2) mice develop a form of progressive pigmentary glaucoma with increasing age. We have compared retinal cell populations of D2 mice with those in control C57BL/6J mice to provide information on retinal histopathology in the D2 mouse. The D2 mouse retina is characterized by a reduction in retinal thickness caused mainly by a thinning of the inner retinal layers. Immunocytochemical staining for specific inner retinal neuronal markers, viz., calbindin for horizontal cells; protein kinase C (PKC) and recoverin for bipolar cells, glycine, γ-aminobutyric acid (GABA), choline acetyltransferase (ChAT), and nitric oxide synthase (NOS) for amacrine cells, and osteopontin (OPN) for ganglion cells, was performed to detect preferentially affected neurons in the D2 mouse retina. Calbindin, PKC, and recoverin immunoreactivities were not significantly altered. Amacrine cells immunoreactive for GABA, ChAT, and OPN were markedly decreased in number, whereas NOS-immunoreactive amacrine cells increased in number. However, no changes were observed in the population of glycine-immunoreactive amacrine cells. These findings indicate a significant loss of retinal ganglion and some amacrine cells, whereas glycinergic amacrine cells, horizontal, and bipolar cells are almost unaffected in the D2 mouse. The reduction in amacrine cells appears to be attributable to a loss of GABAergic and particularly cholinergic amacrine cells. The increase in nitrergic neurons with the consequent increase in NOS and NO may be important in the changes in the retinal organization that lead to glaucomain D2 mice. Thus, the D2 mouse retina represents a useful model for studying the pathogenesis of glaucoma and mechanisms of retinal neuronal death and for evaluating neuroprotection strategies.






Similar content being viewed by others
References
Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, John SWM (2002) Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet 30:81–85
Avila MY, Mitchell CH, Stone RA, Civan MM (2003) Noninvasive assessment of aqueous humor turnover in the mouse eye. Invest Ophthalmol Vis Sci 44:722–727
Bayer AU, Neuhardt T, May AC, Martus P, Maag KP, Brodie S, Lütjen-Drecoll E, Podos SM, Mittag T (2001) Retinal morphology and ERG response in the DBA/2NNia mouse model of angle-closure glaucoma. Invest Ophthalmol Vis Sci 42:1258–1265
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Brooks DE, Garcia GA, Dreyer EB, Zurakowski D, Franco-Bourland RE (1997) Vitreous body glutamate concentration in dogs with glaucoma. Am J Vet Res 58:864–867
Chan HL, Brown B (1999) Multifocal ERG changes in glaucoma. Ophthalmic Physiol Opt 19:306–316
Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova OV, Roderick TH, Heckenlively JR, Davisson MT, John SWM (1999) Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet 21:405–409
Chun MH, Kim IB, Ju WK, Kim KY, Lee MY, Joo CK, Chung JW (1999) Horizontal cells of the rat retina are resistant to degenerative processes induced by ischemia-reperfusion. Neurosci Lett 260:125–128
Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L (2000) Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci 41:2205–2211
Dawson VL, Dawson TM (1998) Nitric oxide in neurodegeneration. Prog Brain Res 118:215–229
Dawson TM, Bredt DS, Fotuhi M, Hwang PM, Snyder SH (1991) Nitric oxide synthase and neuronal NADPH diaphorase are identical in brain and peripheral tissues. Proc Natl Acad Sci USA 88:7797–7801
Dreyer EB, Grosskreutz CL (1997) Excitatory mechanisms in retinal ganglion cell death in primary open angle glaucoma (POAG). Clin Neurosci 4:270–273
Dreyer EB, Zhang D, Lipton SA (1995) Transcriptional or translational inhibition blocks low dose NMDA-mediated cell death. NeuroReport 6:942–944
Dreyer EB, Zurakowski D, Schumer RA, Podos SM, Lipton SA (1996) Elevated glutamate levels in the vitreous body of humans and monkeys with glaucoma. Arch Ophthalmol 114:299–305
Evans K, Bird AC (1996) The genetics of complex ophthalmic disorders. Br J Ophthalmol 80:763–768
Franco-Bourland RE, Guizar-Sahagun G, Garcia GA, Odor-Morales A, Alvarez A, Esquivel F, Rodriguez S (1998) Retinal vulnerability to glutamate excitotoxicity in canine glaucoma: induction of neuronal nitric oxide synthase in retinal ganglion cells. Proc West Pharmacol Soc 41:201–204
Freed MA (1992) GABAergic circuits in the mammalian retina. Prog Brain Res 90:107–131
Ghosh KK, Bujan S, Haverkamp S, Feigenspan A, Wässle H (2004) Types of bipolar cells in the mouse retina. J Comp Neurol 469:70–82
Glovinsky Y, Quigley HA, Dunkelberger GR (1991) Retinal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 32:484–491
Glovinsky Y, Quigley HA, Pease ME (1993) Foveal ganglion cell loss is size dependent in experimental glaucoma. Invest Ophthalmol Vis Sci 34:395–400
Gwon JS, Ju WK, Park SJ, Kim IB, Lee MY, Oh SJ, Chun MH (2001) The regulatory expression of neuronal nitric oxide synthase in the ischemic rat retina. NeuroReport 12:3385–3389
Haverkamp S, Wässle H (2000) Immunocytochemical analysis of the mouse retina. J Comp Neurol 424:1–23
Haverkamp S, Ghosh KK, Hirano AA, Wässle H (2003) Immunocytochemical description of five bipolar cell types of the mouse retina. J Comp Neurol 455:463–476
Hood DC, Greenstein VC, Holopigian K, Bauer R, Firoz B, Liebmann JM, Odel JG, Ritch R (2000) An attempt to detect glaucomatous damage to the inner retina with the multifocal ERG. Invest Ophthalmol Vis Sci 41:1570–1579
Jampel HD, Nickells R, Zack DJ (1996) Glaucoma. In: Rimoin DL, Connor JM, Pteritz RE (eds) Principles and practice of medical genetics. Churchill Livingstone, New York, pp 2505–2521
Janssen P, Naskar R, Moore S, Thanos S, Thiel HJ (1996) Evidence for glaucoma-induced horizontal cell alteration in the human retina. Ger J Ophthalmol 5:378–385
John SWM, Smith RS, Savinova OV, Hawes NL, Chang B, Turnbull D, Davisson M, Roderick TH, Heckenlively JR (1998) Essential iris atrophy, pigment dispersion, and glaucoma in DBA/2J mice. Invest Ophthalmol Vis Sci 39:951–962
John SWM, Anderson MG, Smith RS (1999) Mouse genetics: a tool to help unlock the mechanisms of glaucoma. J Glaucoma 8:400–412
Ju WK, Kim KY, Cha JH, Kim IB, Lee MY, Oh SJ, Chung JW, Chun MH (2000) Ganglion cells of the rat retina show osteopontin-like immunoreactivity. Brain Res 852:217–220
Kendell KR, Quigley HA, Kerrigan LA, Pease ME, Quigley EN (1995) Primary open-angle glaucoma is not associated with photoreceptor loss. Invest Ophthalmol Vis Sci 36:200–205
Kerrigan LA, Zack DJ, Quigley HA, Smith SD, Pease ME (1997) TUNEL-positive ganglion cells in human primary open-angle glaucoma. Arch Ophthalmol 115:1031–1035
Kim IB, Lee EJ, Kim KY, Ju WK, Oh SJ, Joo CK, Chun MH (1999) Immunocytochemical localization of nitric oxide synthase in the mammalian retina. Neurosci Lett 267:193–196
Kim IB, Oh SJ, Chun MH (2000) Neuronal nitric oxide synthase immunoreactive neurons in the mammalian retina. Microsc Res Tech 50:112–123
Korth M (1997) The value of electrophysiology testing in glaucomatous diseases. J Glaucoma 6:331–343
Lambrecht HG, Koch KW (1992) Recoverin, a novel calcium-binding protein from vertebrate photoreceptors. Biochim Biophys Acta 1160:63–66
Laquis S, Chaudhary P, Sharma SC (1998) The patterns of retinal ganglion cell death in hypertensive eyes. Brain Res 784:100–104
Lee EJ, Kim KY, Gu TH, Moon JI, Kim IB, Lee MY, Oh SJ, Chun MH (2003) Neuronal nitric oxide synthase is expressed in the axotomized ganglion cells of the rat retina. Brain Res 986:174–180
Liesegang TJ (1996) Glaucoma: changing concepts and future directions. Mayo Clin Proc 71:689–694
MacNeil MA, Masland RH (1998) Extreme diversity among amacrine cells: implications for function. Neuron 20:971–982
MacNeil MA, Heussy JK, Dacheux RF, Raviola E, Masland RH (1999) The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J Comp Neurol 413:305–326
Marc RE (1989) The role of glycine in the mammalian retina. Prog Ret Res 8:67–107
Masland RH (2001) Neuronal diversity in the retina. Curr Opin Neurobiol 11:431–436
Massey SC, Redburn DA (1987) Transmitter circuits in the vertebrate retina. Prog Neurobiol 28:55–96
Moroni F, Lombardi G, Pellegrini-Faussone S, Moroni F (1993) Photochemically-induced lesion of the rat retina: a quantitative model for the evaluation of ischemia-induced retinal damage. Vision Res 33:1887–1891
Neufeld AH, Hernandez MR, Gonzalez M (1997) Nitric oxide synthase in the human glaucomatous optic nerve head. Arch Ophthalmol 115:497–503
Osborne NN, Ugarte M, Chao M, Chidlow G, Bae JH, Wood JPM, Nash MS (1999) Neuroprotection in relation to retinal ischemia and relevance to glaucoma. Surv Ophthalmol 43 (Suppl 1):S102–S128
Panda S, Jonas JB (1992) Decreased photoreceptor count in human eyes with secondary angle-closure glaucoma. Invest Ophthalmol Vis Sci 33:2532–2536
Peichl L, Gonzalez-Soriano J (1994) Morphological types of horizontal cell in rodent retinae: a comparison of rat, mouse, gerbil, and guinea pig. Vis Neurosci 11:501–517
Pourcho RG (1996) Neurotransmitters in the retina. Curr Eye Res 15:797–803
Pow DV, Wright LL, Vaney DI (1995) The immunocytochemical detection of amino-acid neurotransmitters in paraformaldehyde-fixed tissues. J Neurosci Methods 56:115–123
Quigley HA (1996) Number of people with glaucoma worldwide. Br J Ophthalmol 80:389–393
Quigley HA, Green WR (1979) The histology of human glaucoma cupping and optic nerve damage: clinicopathologic correlation in 21 eyes. Ophthalmology 86:1803–1830
Quigley HA, Addicks EM, Green WR, Maumenee AE (1981) Optic nerve damage in human glaucoma. II. The site of injury and susceptibility to damage. Arch Ophthalmol 99:635–649
Quigley HA, Sanchez RM, Dunkelberger GR, L’Hernault NL, Baginski TA (1987) Chronic glaucoma selectively damages large optic nerve fibers. Invest Ophthalmol Vis Sci 28:913–920
Quigley HA, Dunkelberger GR, Green WR (1988) Chronic human glaucoma causing selectively greater loss of large optic nerve fibers. Ophthalmology 95:357–363
Quigley HA, Dunkelberger GR, Green WR (1989) Retinal ganglion cell atrophy correlated with automated perimetry in human eyes with glaucoma. Am J Ophthalmol 107:453–464
Raz D, Perlman I, Percicot CL, Lambrou GN, Ofri R (2003) Functional damage to inner and outer retinal cells in experimental glaucoma. Invest Ophthalmol Vis Sci 44:3675–3684
Röhrenbach J, Wässle H, Heizmann CW (1987) Immunocytochemical labelling of horizontal cells in mammalian retina using antibodies against calcium-binding proteins. Neurosci Lett 77:255–260
Schuettauf F, Quinto K, Naskar R, Zurakowski D (2002) Effects of anti-glaucoma medications on ganglion cell survival: the DBA/2J mouse model. Vision Res 42:2333–2337
Spencer WH (1996) Glaucoma. In: Spencer WH (ed) Ophthalmic pathology: an atlas and textbook. Saunders, Philadelphia, pp 438–512
Strettoi E, Masland RH (1996) The number of unidentified amacrine cells in the mammalian retina. Proc Natl Acad Sci USA 93:14906–14911
Vaney DI (1990) The mosaic of amacrine cells in the mammalian retina. Prog Ret Res 9:49–100
Wässle H, Boycott BB (1991) Functional architecture of the mammalian retina. Physiol Rev 71:447–480
Wygnanski T, Desatnik H, Quigley HA, Glovinsky Y (1995) Comparison of ganglion cell loss and cone loss in experimental glaucoma. Am J Ophthalmol 120:184–189
Author information
Authors and Affiliations
Corresponding author
Additional information
Jung-Il Moon and In-Beom Kim contributed equally to this work.
This work was supported by a Korea Research Foundation Grant (FP 0005) and by BK 21 in Korea.
Rights and permissions
About this article
Cite this article
Moon, JI., Kim, IB., Gwon, JS. et al. Changes in retinal neuronal populations in the DBA/2J mouse. Cell Tissue Res 320, 51–59 (2005). https://doi.org/10.1007/s00441-004-1062-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-004-1062-8