Abstract
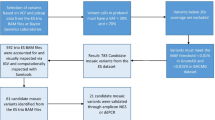
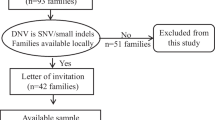
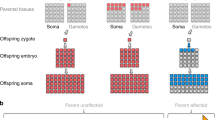
Waardenburg syndrome (WS) is a rare inherited autosomal dominant disorder caused by SOX10, PAX3, MITF, EDNRB, EDN3, and SNAI2. A large burden of pathogenic de novo variants is present in patients with WS, which may be derived from parental mosaicism. Previously, we retrospectively analyzed 90 WS probands with family information. And the frequency of de novo events and parental mosaicism was preliminary investigated in our previous study. In this study, we further explored the occurrence of low-level parental mosaicism in 33 WS families with de novo variants and introduced our procedure of quantifying low-level mosaicism. Mosaic single nucleotide polymorphisms (SNPs) were validated by amplicon-based next-generation sequencing (NGS); copy-number variants (CNVs) were validated by droplet-digital polymerase chain reaction (ddPCR). Molecular validation of low-level mosaicism of WS-causing variants was performed in four families (12.1%, 4/33). These four mosaic variants, comprising three SNVs and one CNV, were identified in SOX10. The rate of parental mosaicism was 25% (4/16) in WS families with de novo SOX10 variants. The lowest allele ratio of a mosaic variant was 2.0% in parental saliva. These de novo WS cases were explained by parental mosaicism conferring an elevated recurrence risk in subsequent pregnancies of parents. Considering its importance in genetic counseling, low-level parental mosaicism should be systematically investigated by personalized sensitive testing. Amplicon-based NGS and ddPCR are recommended to detect and precisely quantify the mosaicism for SNPs and CNVs.




Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the supplementary material of this article.
Code availability
Not applicable.
References
Alicia Gomes MS, Korf B (2018) Genetic testing techniques. Pediatr Cancer Genet 47–64
Campbell IM, Yuan B, Robberecht C et al (2014) Parental somatic mosaicism is underrecognized and influences recurrence risk of genomic disorders. Am J Hum Genet 95(2):173–182
de Lange IM, Koudijs MJ et al (2019) Assessment of parental mosaicism in SCN1A-related epilepsy by single-molecule molecular inversion probes and next-generation sequencing. J Med Genet 56(2):75–80
Domogala DD, Gambin T, Zemet R et al (2021) Stankiewicz, Detection of low-level parental somatic mosaicism for clinically relevant SNVs and indels identified in a large exome sequencing dataset. Hum Genomics 15(1):72
Fu XJ, Nozu K, Kaito H et al (2016) Somatic mosaicism and variant frequency detected by next-generation sequencing in X-linked Alport syndrome. Eur J Hum Genet 24(3):387–391
Hu P, Martinez AF, Kruszka P et al (2019) Low-level parental mosaicism affects the recurrence risk of holoprosencephaly. GIM 21(4):1015–1020
Jiang Q (2019) Sequence characterization of RET in 117 Chinese Hirschsprung disease families identifies a large burden of de novo and parental mosaic mutations. Hum Genomics 14(1):237
Johansson J, Ameur A, Stattin EL et al (2022) Detection of germline mosaicism in fathers of children with intellectual disability syndromes caused by de novo variants. MGGM 2(1):e1880
Liu Q, Grochowski CM, Bi W et al (2020) Quantitative assessment of parental somatic mosaicism for copy-number variant (CNV) deletions. Curr Protoc Hum Genet 106(1):e99
Nayak CS, Glenn I (2003) Worldwide distribution of Waardenburg syndrome. Ann Otol Rhinol Laryngol 112(1):817–820
Notini AJ, Craig JM, White SJ (2008) Copy number variation and mosaicism. Cytogenet Genome Res 123(1–4):270–277
Rahbari R, Wuster A, Lindsay SJ et al (2016) Timing, rates and spectra of human germline mutation. Nat Genet 48(2):126–133
Read AP, Newton VE (1997) Waardenburg syndrome. J Med Genet 34(8):656–665
Shunichi I, Kataoka K et al (2015) Ultra-sensitive detection of the pretreatment EGFR T790M mutation in non-small cell lung cancer patients with an EGFR-activating mutation using droplet digital PCR. Clin Cancer Res 21(15):3552–3560
Summerer A, Schafer E, Mautner VF et al (2019) Ultra-deep amplicon sequencing indicates absence of low-grade mosaicism with normal cells in patients with type-1 NF1 deletions. Hum Genet 138(1):73–81
Sun L, Li X, Shi J et al (2016) Molecular etiology and genotype-phenotype correlation of Chinese Han deaf patients with type I and type II Waardenburg Syndrome. Sci Rep 19(6):35498
Wang G, Li X, Gao X et al (2022) Analysis of genotype-phenotype relationships in 90 Chinese probands with Waardenburg syndrome. Hum Genet 141(3–4):839–852
Xu X, Yang X, Wu Q et al (2015) Amplicon resequencing identified parental mosaicism for approximately 10% of “de novo” SCN1A Mutations in children with Dravet syndrome. Hum Mutat 36(9):861–872
Funding
This work was supported by grants from National Natural Science Foundation of China (82171155) and National Major Scientific Instrument and Equipment Development Project (61827805) to Guojian Wang, National Natural Science Foundation of China (81870713), Beijing Natural Science Foundation (7191011), National Key Research and Development Project (2016YFC1000700, 2016YFC1000704) and National Natural Science Foundation of China (81730029) to Pu Dai, National Key Research and Development Project of China (2016YFC1000706) and National Natural Science Foundation of China (81873704) to Yongyi Yuan, National Natural Science Foundation of China (81870731) to Shasha Huang, National Natural Science Foundation of China (81900953) and Natural Science Foundation of Hainan Province (819MS110) to Mingyu Han.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that no conflict of interest exists.
Ethical approval
Study protocols were performed with the approval of the Ethics Committee of the Chinese PLA General Hospital (approval number S2016-103-01).
Consent for publication
Informed consent was obtained from all probands or guardians for molecular genetic analyses and publication of clinical data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
439_2022_2517_MOESM1_ESM.tif
Fig. S1 BAM files of amplicon-based NGS of DNA extracted from paternal (family 24) blood, saliva, hair follicles, and sperm. (TIF 3312 KB)
439_2022_2517_MOESM2_ESM.tif
Fig. S2 BAM files of amplicon-based NGS of DNA extracted from paternal (family 24) blood, saliva, hair follicles, and sperm. (TIF 3079 KB)
439_2022_2517_MOESM3_ESM.tif
Fig. S3 BAM files of amplicon-based NGS of DNA extracted from paternal (family 24) blood, saliva, hair follicles, and sperm. (TIF 4285 KB)
439_2022_2517_MOESM4_ESM.tif
Fig. S4 BAM files of amplicon-based NGS of DNA extracted from paternal (family 24) blood, saliva, hair follicles, and sperm. (TIF 3183 KB)
439_2022_2517_MOESM7_ESM.tif
Fig. S7 Droplet digital PCR (ddPCR) analysis results in family 33. The CNV deletion junction fragment assessed by ddPCR was ~50% in the proband of family 33, as expected for a normal heterozygous variant; it was 0% in an unrelated control sample. All droplets above the threshold intensity (pink line) were regarded as positive and assigned a value of 1; droplets below the threshold were regarded as negative and assigned a value of 0. These counts enabled calculation of the starting target DNA concentration by statistical analysis of the numbers of positive and negative droplets in a sample. Measurements of breakpoint junction sequences are shown, along with sample types. (TIF 3390 KB)
439_2022_2517_MOESM8_ESM.tif
Fig. S8 Droplet digital PCR (ddPCR) analysis results of family 12. Each panel represents a single ddPCR experiment whereby a DNA sample is segregated into individual droplets and assessed for the presence of CNV deletion using two different fluorophores in Taqman™ assays. The FAM and VIC fluorescence for each droplet is plotted as a data point on each graph. FAM fluorescent signal is plotted on the y-axis and VIC fluorescent signal is plotted on the x-axis. The droplet threshold for each fluorophore used is indicated by the magenta lines, determining whether a droplet is considered positive or negative for either FAM or VIC fluorescence (TIF 1714 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, X., Huang, S., Wang, G. et al. Quantitative assessment of low-level parental mosaicism of SNVs and CNVs in Waardenburg syndrome. Hum Genet 142, 419–430 (2023). https://doi.org/10.1007/s00439-022-02517-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-022-02517-x