Abstract
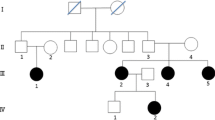
Hirschsprung disease (HSCR) is a neurocristopathy characterized by the absence of enteric ganglia along variable lengths of the intestine. Genetic defects play a major role in HSCR pathogenesis with nearly 50% of patients having a structural or regulatory deficiency in the major susceptibility gene RET. However, complete molecular defects remain poorly characterized in most patients. Here, we performed detailed genetic, molecular, and populational investigations of rare null mutations and modifiers at the RET locus. We first verified the pathogenicity of three RET splice site mutants (c.1879 + 1G > A, c.2607 + 5G > A and c.2608-3C > G) at the RNA level. We also identified significantly higher risk allele (genotype) frequencies, and their over-transmission, from unaffected parents to affected offspring of three functionally independent enhancer variants (rs2506030, rs7069590 and rs2435357, with odd ratios (OR) of 2.09, 2.71 and 7.59, respectively, P < 0.001). These three common variants are in significant (P < 4.64 × 10–186) linkage disequilibrium in the Han Chinese population with ~ 60% of them carrying at least one copy and > 10% with two copies. We show that RET compound inheritance of rare and common variants prevails in 64% (seven out of 11) of Chinese HSCR families. This study supports the idea that common RET variants can modify the penetrance of rare null RET mutations in HSCR, and the combined high susceptibility allele dosage may constitute the unique raised “risk baseline” among the Chinese population.





Similar content being viewed by others
Data availability
The raw WES data are not publicly available due to privacy or ethical restrictions. Processed genetic data generated or analyzed within this study are available upon request.
References
Amiel J, Sproat-Emison E, Garcia-Barcelo M, Lantieri F, Burzynski G, Borrego S, Pelet A, Arnold S, Miao X, Griseri P, Brooks AS, Antinolo G, de Pontual L, Clement-Ziza M, Munnich A, Kashuk C, West K, Wong KK, Lyonnet S, Chakravarti A, Tam PK, Ceccherini I, Hofstra RM, Fernandez R, Hirschsprung Disease Consortium (2008) Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet 45(1):1–14
Bahrami A, Joodi M, Moetamani-Ahmadi M, Maftouh M, Hassanian SM, Ferns GA, Avan A (2018) Genetic background of Hirschsprung disease: a bridge between basic science and clinical application. J Cell Biochem 119(1):28–33
Berto S, Liu Y, Konopka G (2020) Genomics at cellular resolution: insights into cognitive disorders and their evolution. Hum Mol Genet 29(R1):R1–R9. https://doi.org/10.1093/hmg/ddaa117
Chakravarti A (1999) Population genetics–making sense out of sequence. Nat Genet 21(1 Suppl):56–60
Chakravarti A, Kapoor A (2012) Genetics. Mendelian puzzles. Science 335(6071):930–931
Chatterjee S, Kapoor A, Akiyama JA, Auer DR, Lee D, Gabriel S, Berrios C, Pennacchio LA, Chakravarti A (2016) Enhancer variants synergistically drive dysfunction of a gene regulatory network in Hirschsprung disease. Cell 167(2):355–368
Emison ES, Garcia-Barcelo M, Grice EA, Lantieri F, Amiel J, Burzynski G, Fernandez RM, Hao L, Kashuk C, West K, Miao X, Tam PK, Griseri P, Ceccherini I, Pelet A, Jannot AS, de Pontual L, Henrion-Caude A, Lyonnet S, Verheij JB, Hofstra RM, Antiñolo G, Borrego S, McCallion AS, Chakravarti A (2010) Differential contributions of rare and common, coding and noncoding Ret mutations to multifactorial Hirschsprung disease liability. Am J Hum Genet 87(1):60–74
Gao Y, Zhang C, Yuan L, Ling Y, Wang X, Liu C, Pan Y, Zhang X, Ma X, Wang Y, Lu Y, Yuan K, Ye W, Qian J, Chang H, Cao R, Yang X, Ma L, Ju Y, Dai L, Tang Y, Han100K Initiative, Zhang G, Xu S (2020) PGGHan: the Han Chinese genome database and analysis platform. Nucleic Acids Res 48(D1):D971–D976
Gunadi IK, Makhmudi A, Kapoor A (2019) Combined genetic effects of RET and NRG1 susceptibility variants on multifactorial Hirschsprung disease in Indonesia. J Surg Res 233:96–99
Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, Kosmicki JA, Arbelaez J, Cui W, Schwartz GB, Chow ED, Kanterakis E, Gao H, Kia A, Batzoglou S, Sanders SJ, Farh KK (2019) Predicting splicing from primary sequence with deep learning. Cell 176(3):535–548
Jiang Q, Liu F, Miao C, Li Q, Zhang Z, Xiao P, Su L, Yu K, Chen X, Zhang F, Chakravarti A, Li L (2018) RET somatic mutations are underrecognized in Hirschsprung disease. Genet Med 20(7):770–777
Jiang Q, Wang Y, Li Q, Zhang Z, Xiao P, Wang H, Liu N, Wu J, Zhang F, Chakravarti A, Cai W, Li L (2019) Sequence characterization of RET in 117 Chinese Hirschsprung disease families identifies a large burden of de novo and parental mosaic mutations. Orphanet J Rare Dis 14(1):237
Kapoor A, Jiang Q, Chatterjee S, Chakraborty P, Sosa MX, Berrios C, Chakravarti A (2015) Population variation in total genetic risk of Hirschsprung disease from common RET, SEMA3 and NRG1 susceptibility polymorphisms. Hum Mol Genet 24(10):2997–3003
Li R, Liu Y, Hou Y, Gan J, Wu P, Li C (2018) 3D genome and its disorganization in diseases. Cell Biol Toxicol 34(5):351–365
Lord J, Gallone G, Short PJ, McRae JF, Ironfield H, Wynn EH, Gerety SS, He L, Kerr B, Johnson DS, McCann E, Kinning E, Flinter F, Temple IK, Clayton-Smith J, McEntagart M, Lynch SA, Joss S, Douzgou S, Dabir T, Clowes V, McConnell VPM, Lam W, Wright CF, FitzPatrick DR, Firth HV, Barrett JC, Hurles ME, Deciphering Developmental Disorders study (2019) Pathogenicity and selective constraint on variation near splice sites. Genome Res 29(2):159–170
Luzon-Toro B, Villalba-Benito L, Torroglosa A, Fernandez RM, Antinolo G, Borrego S (2020) What is new about the genetic background of Hirschsprung disease? Clin Genet 97(1):114–124
Maston GA, Evans SK, Green MR (2006) Transcriptional regulatory elements in the human genome Annu Rev Genomics. Hum Genet 7:29–59
Mc Laughlin D, Puri P (2015) Familial Hirschsprung’s disease: a systematic review. Pediatr Surg Int 31(8):695–700
Mishra A, Hawkins RD (2017) Three-dimensional genome architecture and emerging technologies: looping in disease. Genome Med 9(1):87
Moore SW (2017) Advances in understanding functional variations in the Hirschsprung disease spectrum (variant Hirschsprung disease). Pediatr Surg Int 33(3):285–298
Pritchard JK, Cox NJ (2002) The allelic architecture of human disease genes: common disease-common variant… or not? Hum Mol Genet 11(20):2417–2423
Spielman RS, McGinnis RE, Ewens WJ (1993) Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM). Am J Hum Genet 52(3):506–516
Stubbington MJT, Rozenblatt-Rosen O, Regev A, Teichmann SA (2017) Single-cell transcriptomics to explore the immune system in health and disease. Science 358(6359):58–63
Tang CS, Gui H, Kapoor A, Kim JH, Luzón-Toro B, Pelet A, Burzynski G, Lantieri F, So MT, Berrios C, Shin HD, Fernández RM, Le TL, Verheij JB, Matera I, Cherny SS, Nandakumar P, Cheong HS, Antiñolo G, Amiel J, Seo JM, Kim DY, Oh JT, Lyonnet S, Borrego S, Ceccherini I, Hofstra RM, Chakravarti A, Kim HY, Sham PC, Tam PK, Garcia-Barceló MM (2016) Trans-ethnic meta-analysis of genome-wide association studies for Hirschsprung disease. Hum Mol Genet 25(23):5265–5275
Tilghman JM, Ling AY, Turner TN, Sosa MX, Krumm N, Chatterjee S, Kapoor A, Coe BP, Nguyen KH, Gupta N, Gabriel S, Eichler EE, Berrios C, Chakravarti A (2019) Molecular genetic anatomy and risk profile of Hirschsprung’s disease. N Engl J Med 380(15):1421–1432
Wang Y, Jiang Q, Cai H, Xu Z, Wu W, Gu B, Li L, Cai W (2020) Genetic variants in RET, ARHGEF3 and CTNNAL1, and relevant interaction networks, contribute to the risk of Hirschsprung disease. Aging (Albany NY) 12(5):4379–4393
Xie X, Shi Q, Wu P, Zhang X, Kambara H, Su J, Yu H, Park SY, Guo R, Ren Q, Zhang S, Xu Y, Silberstein LE, Cheng T, Ma F, Li C, Luo HR (2020) Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat Immunol 21(9):1119–1133
Zhang S, Samocha KE, Rivas MA, Karczewski KJ, Daly E, Schmandt B, Neale BM, MacArthur DG, Daly MJ (2018) Base-specific mutational intolerance near splice sites clarifies the role of nonessential splice nucleotides. Genome Res 28(7):968–974
Acknowledgements
We are grateful to the many patients and their families whose cooperation made this study possible. The English language editing was achieved at American Journal Experts. We also thank Prof. Aravinda Chakravarti from the New York University School of Medicine and Dr. Sen Zhao, Nan Wu from the Peking Union Medical College Hospital for comments on this manuscript.
Funding
This work was made possible by a grant from the Chinese Academy of Medical Sciences Initiative for Innovative Medicine (CAMS-I2M) and the National Natural Science Foundation of China (81771620, 82070532) to Qian Jiang. Yang Wang was supported by the National Natural Science Foundation of China (81670469), Shanghai Municipal Commission of Health and Family Planning (201840028) and the Innovative Research Team of High-level Local Universities in Shanghai. Zhen Zhang was supported by the National Natural Science Foundation of China (81700451). Shuhua Xu was supported by the National Natural Science Foundation of China (91731303, 31771388, 31961130380, and 31711530221), the National Science Fund for Distinguished Young Scholars (31525014), the UK Royal Society-Newton Advanced Fellowship (NAF\R1\191094), Key Research Program of Frontier Sciences (QYZDJ-SSW-SYS009) of the Chinese Academy of Sciences, and the Shanghai Municipal Science and Technology Major Project (2017SHZDZX01). Wei Cai was supported by the National Natural Science Foundation of China (81630039) and Shanghai Key Laboratory of Pediatric Gastroenterology and Nutrition (17DZ2272000). Long Li was supported by grants from the Public Welfare Industry Research Special Foundation of China (201402007).
Author information
Authors and Affiliations
Contributions
QJ and YW prepared the manuscript. QJ and YW performed the genetic analyses and minigene study. HW, QL and ZZ conducted sample acquisition and collected clinical information. YG and SX conducted haplotype frequency analyses in the Han Chinese population. WC and LL supervised the study and edited the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical approval
Patient sampling was conducted through the Hirschsprung Disease Consortium of China (HDCC) spanning two clinical centers across North and South China. This study was approved by the medical ethics committee of the Capital Institute of Pediatrics (SHERLL 2013039) and Xinhua Hospital (XHEC-C-2016-263) and was carried out according to the Declaration of Helsinki.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Subjects or their legal representatives participating in this study provided written consent for publication of the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jiang, Q., Wang, Y., Gao, Y. et al. RET compound inheritance in Chinese patients with Hirschsprung disease: lack of penetrance from insufficient gene dysfunction. Hum Genet 140, 813–825 (2021). https://doi.org/10.1007/s00439-020-02247-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-020-02247-y