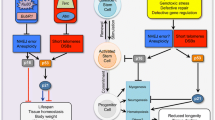
Abstract
DNA damage is one of the most consistent cellular process proposed to contribute to aging. The maintenance of genomic and epigenomic integrity is critical for proper function of cells and tissues throughout life, and this homeostasis is under constant strain from both extrinsic and intrinsic insults. Considering the relationship between lifespan and genotoxic burden, it is plausible that the longest-lived cellular populations would face an accumulation of DNA damage over time. Tissue-specific stem cells are multipotent populations residing in localized niches and are responsible for maintaining all lineages of their resident tissue/system throughout life. However, many of these stem cells are impacted by genotoxic stress. Several factors may dictate the specific stem cell population response to DNA damage, including the niche location, life history, and fate decisions after damage accrual. This leads to differential handling of DNA damage in different stem cell compartments. Given the importance of adult stem cells in preserving normal tissue function during an individual’s lifetime, DNA damage sensitivity and accumulation in these compartments could have crucial implications for aging. Despite this, more support for direct functional effects driven by accumulated DNA damage in adult stem cell compartments is needed. This review will present current evidence for the accumulation and potential influence of DNA damage in adult tissue-specific stem cells and propose inquiry directions that could benefit individual healthspan.

Similar content being viewed by others
References
Acharya MM, Lan ML, Kan VH, Patel NH, Giedzinski E, Tseng BP, Limoli CL (2010) Consequences of ionizing radiation-induced damage in human neural stem cells. Free Radic Biol Med 49:1846–1855
Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z (2009) Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J Neurosci 29:4408–4419
Ahlqvist KJ, Hamalainen RH, Yatsuga S, Uutela M, Terzioglu M, Gotz A, Forsstrom S, Salven P, Angers-Loustau A, Kopra OH, Tyynismaa H, Larsson NG, Wartiovaara K, Prolla T, Trifunovic A, Suomalainen A (2012) Somatic progenitor cell vulnerability to mitochondrial DNA mutagenesis underlies progeroid phenotypes in Polg mutator mice. Cell Metab 15:100–109
Ahmed EA, van der Vaart A, Barten A, Kal HB, Chen J, Lou Z, Minter-Dykhouse K, Bartkova J, Bartek J, de Boer P, de Rooij DG (2007) Differences in DNA double strand breaks repair in male germ cell types: lessons learned from a differential expression of Mdc1 and 53BP1. DNA Repair (Amst) 6:1243–1254
Allen DM, van Praag H, Ray J, Weaver Z, Winrow CJ, Carter TA, Braquet R, Harrington E, Ried T, Brown KD, Gage FH, Barlow C (2001) Ataxia telangiectasia mutated is essential during adult neurogenesis. Genes Dev 15:554–566
Allsopp RC, Morin GB, Horner JW, DePinho R, Harley CB, Weissman IL (2003) Effect of TERT over-expression on the long-term transplantation capacity of hematopoietic stem cells. Nat Med 9:369–371
Alshahrani S, Agarwal A, Assidi M, Abuzenadah AM, Durairajanayagam D, Ayaz A, Sharma R, Sabanegh E (2014) Infertile men older than 40 years are at higher risk of sperm DNA damage. Reprod Biol Endocrinol 12:103
Alves H, Munoz-Najar U, De Wit J, Renard AJ, Hoeijmakers JH, Sedivy JM, Van Blitterswijk C, De Boer J (2010) A link between the accumulation of DNA damage and loss of multi-potency of human mesenchymal stromal cells. J Cell Mol Med 14:2729–2738
Ameur A, Stewart JB, Freyer C, Hagstrom E, Ingman M, Larsson NG, Gyllensten U (2011) Ultra-deep sequencing of mouse mitochondrial DNA: mutational patterns and their origins. PLoS Genet 7:e1002028
Anjos-Afonso F, Loizou JI, Bradburn A, Kanu N, Purewal S, Da Costa C, Bonnet D, Behrens A (2016) Perturbed hematopoiesis in mice lacking ATMIN. Blood 128:2017–2021
Antunes DM, Kalmbach KH, Wang F, Dracxler RC, Seth-Smith ML, Kramer Y, Buldo-Licciardi J, Kohlrausch FB, Keefe DL (2015) A single-cell assay for telomere DNA content shows increasing telomere length heterogeneity, as well as increasing mean telomere length in human spermatozoa with advancing age. J Assist Reprod Genet 32:1685–1690
Apple DM, Mahesula S, Fonseca RS, Zhu C, Kokovay E (2019) Calorie restriction protects neural stem cells from age-related deficits in the subventricular zone. Aging (Albany NY) 11:115–126
Avagyan S, Churchill M, Yamamoto K, Crowe JL, Li C, Lee BJ, Zheng T, Mukherjee S, Zha S (2014) Hematopoietic stem cell dysfunction underlies the progressive lymphocytopenia in XLF/Cernunnos deficiency. Blood 124:1622–1625
Bahrami A, Ro JY, Ayala AG (2007) An overview of testicular germ cell tumors. Arch Pathol Lab Med 131:1267–1280
Bailey KJ, Maslov AY, Pruitt SC (2004) Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell 3:391–397
Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM (2004) BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet 36:744–749
Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM (2011) Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479:232–236
Barcia RN, Santos JM, Filipe M, Teixeira M, Martins JP, Almeida J, Agua-Doce A, Almeida SC, Varela A, Pohl S, Dittmar KE, Calado S, Simoes SI, Gaspar MM, Cruz ME, Lindenmaier W, Graca L, Cruz H, Cruz PE (2015) What makes umbilical cord tissue-derived mesenchymal stromal cells superior immunomodulators when compared to bone marrow derived mesenchymal stromal cells? Stem Cells Int 2015:583984
Beerman I (2017) Accumulation of DNA damage in the aged hematopoietic stem cell compartment. Semin Hematol 54:12–18
Beerman I, Rossi DJ (2015) ‘Epigenetic control of stem cell potential during homeostasis, aging, and disease. Cell Stem Cell 16:613–625
Beerman I, Maloney WJ, Weissmann IL, Rossi DJ (2010) Stem cells and the aging hematopoietic system. Curr Opin Immunol 22:500–506
Beerman I, Bock C, Garrison BS, Smith ZD, Gu H, Meissner A, Rossi DJ (2013) Proliferation-dependent alterations of the DNA methylation landscape underlie hematopoietic stem cell aging. Cell Stem Cell 12:413–425
Beerman I, Seita J, Inlay MA, Weissman IL, Rossi DJ (2014) Quiescent hematopoietic stem cells accumulate DNA damage during aging that is repaired upon entry into cell cycle. Cell Stem Cell 15:37–50
Bernitz JM, Kim HS, MacArthur B, Sieburg H, Moore K (2016) Hematopoietic stem cells count and remember self-renewal divisions. Cell 167(1296–309):e10
Bigot A, Duddy WJ, Ouandaogo ZG, Negroni E, Mariot V, Ghimbovschi S, Harmon B, Wielgosik A, Loiseau C, Devaney J, Dumonceaux J, Butler-Browne G, Mouly V, Duguez S (2015) Age-associated methylation suppresses SPRY1, leading to a failure of re-quiescence and loss of the reserve stem cell pool in elderly muscle. Cell Rep 13:1172–1182
Biteau B, Hochmuth CE, Jasper H (2008) JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell 3:442–455
Blanpain C, Fuchs E (2006) Epidermal stem cells of the skin. Annu Rev Cell Dev Biol 22:339–373
Blokzijl F, de Ligt J, Jager M, Sasselli V, Roerink S, Sasaki N, Huch M, Boymans S, Kuijk E, Prins P, Nijman IJ, Martincorena I, Mokry M, Wiegerinck CL, Middendorp S, Sato T, Schwank G, Nieuwenhuis EE, Verstegen MM, van der Laan LJ, de Jonge J, IJzermans JN, Vries RG, van de Wetering m, Stratton MR, Clevers H, Cuppen E, van Boxtel R (2016) Tissue-specific mutation accumulation in human adult stem cells during life. Nature 538:260–264
Booth C, Tudor GL, Katz BP, MacVittie TJ (2015) The delayed effects of acute radiation syndrome: evidence of long-term functional changes in the clonogenic cells of the small intestine. Health Phys 109:399–413
Borodkina A, Shatrova A, Abushik P, Nikolsky N, Burova E (2014) Interaction between ROS dependent DNA damage, mitochondria and p38 MAPK underlies senescence of human adult stem cells. Aging (Albany NY) 6:481–495
Brosh RM Jr, Bellani M, Liu Y, Seidman MM (2017) Fanconi anemia: a DNA repair disorder characterized by accelerated decline of the hematopoietic stem cell compartment and other features of aging. Ageing Res Rev 33:67–75
Bugge M, Collins A, Petersen MB, Fisher J, Brandt C, Hertz JM, Tranebjaerg L, de Lozier-Blanchet C, Nicolaides P, Brondum-Nielsen K, Morton N, Mikkelsen M (1998) Non-disjunction of chromosome 18. Hum Mol Genet 7:661–669
Busque L, Buscarlet M, Mollica L, Levine RL (2018) Concise review: age-related clonal hematopoiesis: stem cells tempting the devil. Stem Cells 36:1287–1294
Carruthers RD, Ahmed SU, Ramachandran S, Strathdee K, Kurian KM, Hedley A, Gomez-Roman N, Kalna G, Neilson M, Gilmour L, Stevenson KH, Hammond EM, Chalmers AJ (2018) Replication stress drives constitutive activation of the DNA damage response and radioresistance in glioblastoma stem-like cells. Cancer Res 78:5060–5071
Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL, Longaker MT (2015) Identification and specification of the mouse skeletal stem cell. Cell 160:285–298
Chan CKF, Gulati GS, Sinha R, Tompkins JV, Lopez M, Carter AC, Ransom RC, Reinisch A, Wearda T, Murphy M, Brewer RE, Koepke LS, Marecic O, Manjunath A, Seo EY, Leavitt T, Lu WJ, Nguyen A, Conley SD, Salhotra A, Ambrosi TH, Borrelli MR, Siebel T, Chan K, Schallmoser K, Seita J, Sahoo D, Goodnough H, Bishop J, Gardner M, Majeti R, Wan DC, Goodman S, Weissman IL, Chang HY, Longaker MT (2018) Identification of the human skeletal stem cell. Cell 175(43–56):e21
Chen Q, Liu K, Robinson AR, Clauson CL, Blair HC, Robbins PD, Niedernhofer LJ, Ouyang H (2013) DNA damage drives accelerated bone aging via an NF-kappaB-dependent mechanism. J Bone Miner Res 28:1214–1228
Chen H, Liu X, Zhu W, Chen H, Hu X, Jiang Z, Xu Y, Wang L, Zhou Y, Chen P, Zhang N, Hu D, Zhang L, Wang Y, Xu Q, Wu R, Yu H, Wang J (2014) SIRT1 ameliorates age-related senescence of mesenchymal stem cells via modulating telomere shelterin. Front Aging Neurosci 6:103
Chen J, Bryant MA, Dent JJ, Sun Y, Desierto MJ, Young NS (2015) Hematopoietic lineage skewing and intestinal epithelia degeneration in aged mice with telomerase RNA component deletion. Exp Gerontol 72:251–260
Cheung HH, Liu X, Canterel-Thouennon L, Li L, Edmonson C, Rennert OM (2014) Telomerase protects werner syndrome lineage-specific stem cells from premature aging. Stem Cell Rep 2:534–546
Cho JS, Kook SH, Robinson AR, Niedernhofer LJ, Lee BC (2013) Cell autonomous and nonautonomous mechanisms drive hematopoietic stem/progenitor cell loss in the absence of DNA repair. Stem Cells 31:511–525
Choi J, Rakhilin N, Gadamsetty P, Joe DJ, Tabrizian T, Lipkin SM, Huffman DM, Shen X, Nishimura N (2018) Intestinal crypts recover rapidly from focal damage with coordinated motion of stem cells that is impaired by aging. Sci Rep 8:10989
Colla S, Ong DS, Ogoti Y, Marchesini M, Mistry NA, Clise-Dwyer K, Ang SA, Storti P, Viale A, Giuliani N, Ruisaard K, Ganan Gomez I, Bristow CA, Estecio M, Weksberg DC, Ho YW, Hu B, Genovese G, Pettazzoni P, Multani AS, Jiang S, Hua S, Ryan MC, Carugo A, Nezi L, Wei Y, Yang H, D’Anca M, Zhang L, Gaddis S, Gong T, Horner JW, Heffernan TP, Jones P, Cooper LJ, Liang H, Kantarjian H, Wang YA, Chin L, Bueso-Ramos C, Garcia-Manero G, DePinho RA (2015) Telomere dysfunction drives aberrant hematopoietic differentiation and myelodysplastic syndrome. Cancer Cell 27:644–657
Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA (2005) Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433:760–764
Corenblum MJ, Ray S, Remley QW, Long M, Harder B, Zhang DD, Barnes CA, Madhavan L (2016) Reduced Nrf2 expression mediates the decline in neural stem cell function during a critical middle-age period. Aging Cell 15:725–736
Cousin W, Ho ML, Desai R, Tham A, Chen RY, Kung S, Elabd C, Conboy IM (2013) Regenerative capacity of old muscle stem cells declines without significant accumulation of DNA damage. PLoS One 8:e63528
Cui H, Tang D, Garside GB, Zeng T, Wang Y, Tao Z, Zhang L, Tao S (2019) Wnt signaling mediates the aging-induced differentiation impairment of intestinal stem cells. Stem Cell Rev 15(3):448–455
Di Foggia V, Zhang X, Licastro D, Gerli MF, Phadke R, Muntoni F, Mourikis P, Tajbakhsh S, Ellis M, Greaves LC, Taylor RW, Cossu G, Robson LG, Marino S (2014) Bmi1 enhances skeletal muscle regeneration through MT1-mediated oxidative stress protection in a mouse model of dystrophinopathy. J Exp Med 211:2617–2633
Di Giacomo M, Barchi M, Baudat F, Edelmann W, Keeney S, Jasin M (2005) Distinct DNA-damage-dependent and -independent responses drive the loss of oocytes in recombination-defective mouse mutants. Proc Natl Acad Sci USA 102:737–742
Diao D, Wang H, Li T, Shi Z, Jin X, Sperka T, Zhu X, Zhang M, Yang F, Cong Y, Shen L, Zhan Q, Yan J, Song Z, Ju Z (2018) Telomeric epigenetic response mediated by Gadd45a regulates stem cell aging and lifespan. EMBO Rep 19(10):e45494. https://doi.org/10.15252/embr.201745494
Diderich KE, Nicolaije C, Priemel M, Waarsing JH, Day JS, Brandt RM, Schilling AF, Botter SM, Weinans H, van der Horst GT, Hoeijmakers JH, van Leeuwen JP (2012) Bone fragility and decline in stem cells in prematurely aging DNA repair deficient trichothiodystrophy mice. Age (Dordr) 34:845–861
Didier N, Hourde C, Amthor H, Marazzi G, Sassoon D (2012) Loss of a single allele for Ku80 leads to progenitor dysfunction and accelerated aging in skeletal muscle. EMBO Mol Med 4:910–923
Dong CM, Wang XL, Wang GM, Zhang WJ, Zhu L, Gao S, Yang DJ, Qin Y, Liang QJ, Chen YL, Deng HT, Ning K, Liang AB, Gao ZL, Xu J (2014) A stress-induced cellular aging model with postnatal neural stem cells. Cell Death Dis 5:e1116
Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, Koulakov A, Enikolopov G (2011) Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8:566–579
Feng X, Xing J, Feng G, Huang D, Lu X, Liu S, Tan W, Li L, Gu Z (2014) p16(INK4A) mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech Ageing Dev 141–142:46–55
Finch Caleb, Kirkwood TBL (2000) Chance, development, and aging. Oxford University Press, New York
Fisher JM, Harvey JF, Morton NE, Jacobs PA (1995) Trisomy 18: studies of the parent and cell division of origin and the effect of aberrant recombination on nondisjunction. Am J Hum Genet 56:669–675
Flach J, Bakker ST, Mohrin M, Conroy PC, Pietras EM, Reynaud D, Alvarez S, Diolaiti ME, Ugarte F, Forsberg EC, Le Beau MM, Stohr BA, Mendez J, Morrison CG, Passegue E (2014) Replication stress is a potent driver of functional decline in ageing haematopoietic stem cells. Nature 512:198–202
Fox RG, Magness S, Kujoth GC, Prolla TA, Maeda N (2012) Mitochondrial DNA polymerase editing mutation, PolgD257A, disturbs stem-progenitor cell cycling in the small intestine and restricts excess fat absorption. Am J Physiol Gastrointest Liver Physiol 302:G914–G924
Franceschi C, Bonafe M, Valensin S (2000) Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine 18:1717–1720
Franco I, Johansson A, Olsson K, Vrtacnik P, Lundin P, Helgadottir HT, Larsson M, Revechon G, Bosia C, Pagnani A, Provero P, Gustafsson T, Fischer H, Eriksson M (2018) Somatic mutagenesis in satellite cells associates with human skeletal muscle aging. Nat Commun 9:800
Fuchs E (2008) Skin stem cells: rising to the surface. J Cell Biol 180:273–284
Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, Vuong J, Jacob S, Muralidhar V, Robertson AA, Cooper MA, Andres V, Hirschi KK, Martin KA, Walsh K (2017) Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science 355:842–847
Gage FH, Temple S (2013) Neural stem cells: generating and regenerating the brain. Neuron 80:588–601
Galderisi U, Helmbold H, Squillaro T, Alessio N, Komm N, Khadang B, Cipollaro M, Bohn W, Giordano A (2009) In vitro senescence of rat mesenchymal stem cells is accompanied by downregulation of stemness-related and DNA damage repair genes. Stem Cells Dev 18:1033–1042
Garcia-Prat L, Martinez-Vicente M, Perdiguero E, Ortet L, Rodriguez-Ubreva J, Rebollo E, Ruiz-Bonilla V, Gutarra S, Ballestar E, Serrano AL, Sandri M, Munoz-Canoves P (2016) Autophagy maintains stemness by preventing senescence. Nature 529:37–42
Ge ZJ, Schatten H, Zhang CL, Sun QY (2015) Oocyte ageing and epigenetics. Reproduction 149:R103–R114
Gervais L, Bardin AJ (2017) Tissue homeostasis and aging: new insight from the fly intestine. Curr Opin Cell Biol 48:97–105
Giangreco A, Qin M, Pintar JE, Watt FM (2008) Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell 7:250–259
Gierut JJ, Lyons J, Shah MS, Genetti C, Breault DT, Haigis KM (2015) Oncogenic K-Ras promotes proliferation in quiescent intestinal stem cells. Stem Cell Res 15:165–171
Gladyshev VN (2013) The origin of aging: imperfectness-driven non-random damage defines the aging process and control of lifespan. Trends Genet 29:506–512
Gnani D, Crippa S, Della Volpe L, Rossella V, Conti A, Lettera E, Rivis S, Ometti M, Fraschini G, Bernardo ME, Di Micco R (2019) An early-senescence state in aged mesenchymal stromal cells contributes to hematopoietic stem and progenitor cell clonogenic impairment through the activation of a pro-inflammatory program. Aging Cell 18(3):e12933. https://doi.org/10.1111/acel.12933
Goriely A, Wilkie AO (2012) Paternal age effect mutations and selfish spermatogonial selection: causes and consequences for human disease. Am J Hum Genet 90:175–200
Goriely A, McVean GA, Rojmyr M, Ingemarsson B, Wilkie AO (2003) Evidence for selective advantage of pathogenic FGFR2 mutations in the male germ line. Science 301:643–646
Goriely A, McVean GA, van Pelt AM, O’Rourke AW, Wall SA, de Rooij DG, Wilkie AO (2005) Gain-of-function amino acid substitutions drive positive selection of FGFR2 mutations in human spermatogonia. Proc Natl Acad Sci USA 102:6051–6056
Greaves LC, Preston SL, Tadrous PJ, Taylor RW, Barron MJ, Oukrif D, Leedham SJ, Deheragoda M, Sasieni P, Novelli MR, Jankowski JA, Turnbull DM, Wright NA, McDonald SA (2006) Mitochondrial DNA mutations are established in human colonic stem cells, and mutated clones expand by crypt fission. Proc Natl Acad Sci USA 103:714–719
Greaves LC, Nooteboom M, Elson JL, Tuppen HA, Taylor GA, Commane DM, Arasaradnam RP, Khrapko K, Taylor RW, Kirkwood TB, Mathers JC, Turnbull DM (2014) Clonal expansion of early to mid-life mitochondrial DNA point mutations drives mitochondrial dysfunction during human ageing. PLoS Genet 10:e1004620
Gutierrez-Martinez P, Hogdal L, Nagai M, Kruta M, Singh R, Sarosiek K, Nussenzweig A, Beerman I, Letai A, Rossi DJ (2018) Diminished apoptotic priming and ATM signalling confer a survival advantage onto aged haematopoietic stem cells in response to DNA damage. Nat Cell Biol 20:413–421
Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, Ko MS (2004) Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 13:2263–2278
Hare I, Gencheva M, Evans R, Fortney J, Piktel D, Vos JA, Howell D, Gibson LF (2016) In vitro expansion of bone marrow derived mesenchymal stem cells alters DNA double strand break repair of etoposide induced DNA damage. Stem Cells Int 2016:8270464
Harman D (1956) Aging: a theory based on free radical and radiation chemistry. J Gerontol 11:298–300
Harrison DE (1972) Normal function of transplanted mouse erythrocyte precursors for 21 months beyond donor life spans. Nat New Biol 237:220–222
Hassold T, Merrill M, Adkins K, Freeman S, Sherman S (1995) Recombination and maternal age-dependent nondisjunction: molecular studies of trisomy 16. Am J Hum Genet 57:867–874
Hasty P, Campisi J, Hoeijmakers J, van Steeg H, Vijg J (2003) Aging and genome maintenance: lessons from the mouse? Science 299:1355–1359
Hishiya A, Ito M, Aburatani H, Motoyama N, Ikeda K, Watanabe K (2005) Ataxia telangiectasia mutated (Atm) knockout mice as a model of osteopenia due to impaired bone formation. Bone 37:497–503
Ho TT, Warr MR, Adelman ER, Lansinger OM, Flach J, Verovskaya EV, Figueroa ME, Passegue E (2017) Autophagy maintains the metabolism and function of young and old stem cells. Nature 543:205–210
Hochmuth CE, Biteau B, Bohmann D, Jasper H (2011) Redox regulation by Keap1 and Nrf2 controls intestinal stem cell proliferation in Drosophila. Cell Stem Cell 8:188–199
Hou J, Han ZP, Jing YY, Yang X, Zhang SS, Sun K, Hao C, Meng Y, Yu FH, Liu XQ, Shi YF, Wu MC, Zhang L, Wei LX (2013) Autophagy prevents irradiation injury and maintains stemness through decreasing ROS generation in mesenchymal stem cells. Cell Death Dis 4:e844
Hu Z, Fu YX, Greenberg AJ, Wu CI, Zhai W (2013) Age-dependent transition from cell-level to population-level control in murine intestinal homeostasis revealed by coalescence analysis. PLoS Genet 9:e1003326
Hunt P, Hassold T (2010) Female meiosis: coming unglued with age. Curr Biol 20:R699–R702
Igarashi M, Miura M, Williams E, Jaksch F, Kadowaki T, Yamauchi T, Guarente L (2019) NAD+ supplementation rejuvenates aged gut adult stem cells. Aging Cell 18(3):e12935. https://doi.org/10.1111/acel.12935
Inagaki A, Roset R, Petrini JH (2016) Functions of the MRE11 complex in the development and maintenance of oocytes. Chromosoma 125:151–162
Inomata K, Aoto T, Binh NT, Okamoto N, Tanimura S, Wakayama T, Iseki S, Hara E, Masunaga T, Shimizu H, Nishimura EK (2009) Genotoxic stress abrogates renewal of melanocyte stem cells by triggering their differentiation. Cell 137:1088–1099
Ishii K, Ishiai M, Morimoto H, Kanatsu-Shinohara M, Niwa O, Takata M, Shinohara T (2014) The Trp53-Trp53inp1-Tnfrsf10b pathway regulates the radiation response of mouse spermatogonial stem cells. Stem Cell Rep 3:676–689
Jacques TS, Swales A, Brzozowski MJ, Henriquez NV, Linehan JM, Mirzadeh Z, O’Malley C, Naumann H, Alvarez-Buylla A, Brandner S (2010) Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J 29:222–235
Jaiswal S, Fontanillas P, Flannick J, Manning A, Grauman PV, Mar BG, Lindsley RC, Mermel CH, Burtt N, Chavez A, Higgins JM, Moltchanov V, Kuo FC, Kluk MJ, Henderson B, Kinnunen L, Koistinen HA, Ladenvall C, Getz G, Correa A, Banahan BF, Gabriel S, Kathiresan S, Stringham HM, McCarthy MI, Boehnke M, Tuomilehto J, Haiman C, Groop L, Atzmon G, Wilson JG, Neuberg D, Altshuler D, Ebert BL (2014) Age-related clonal hematopoiesis associated with adverse outcomes. N Engl J Med 371:2488–2498
Janich P, Pascual G, Merlos-Suarez A, Batlle E, Ripperger J, Albrecht U, Cheng HY, Obrietan K, Di Croce L, Benitah SA (2011) The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 480:209–214
Kalamakis G, Brune D, Ravichandran S, Bolz J, Fan W, Ziebell F, Stiehl T, Catala-Martinez F, Kupke J, Zhao S, Llorens-Bobadilla E, Bauer K, Limpert S, Berger B, Christen U, Schmezer P, Mallm JP, Berninger B, Anders S, Del Sol A, Marciniak-Czochra A, Martin-Villalba A (2019) Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell 176(1407–19):e14
Kaschutnig P, Bogeska R, Walter D, Lier A, Huntscha S, Milsom MD (2015) The Fanconi anemia pathway is required for efficient repair of stress-induced DNA damage in haematopoietic stem cells. Cell Cycle 14:2734–2742
Kashiwagi H, Shiraishi K, Sakaguchi K, Nakahama T, Kodama S (2018) Repair kinetics of DNA double-strand breaks and incidence of apoptosis in mouse neural stem/progenitor cells and their differentiated neurons exposed to ionizing radiation. J Radiat Res 59:261–271
Keefe DL, Marquard K, Liu L (2006) The telomere theory of reproductive senescence in women. Curr Opin Obstet Gynecol 18:280–285
Kempf SJ, Casciati A, Buratovic S, Janik D, von Toerne C, Ueffing M, Neff F, Moertl S, Stenerlow B, Saran A, Atkinson MJ, Eriksson P, Pazzaglia S, Tapio S (2014) The cognitive defects of neonatally irradiated mice are accompanied by changed synaptic plasticity, adult neurogenesis and neuroinflammation. Mol Neurodegener 9:57
Kempf SJ, Janik D, Barjaktarovic Z, Braga-Tanaka I 3rd, Tanaka S, Neff F, Saran A, Larsen MR, Tapio S (2016) Chronic low-dose-rate ionising radiation affects the hippocampal phosphoproteome in the ApoE−/− Alzheimer’s mouse model. Oncotarget 7:71817–71832
Kenyon J, Fu P, Lingas K, Thomas E, Saurastri A, Santos Guasch G, Wald D, Gerson SL (2012) Humans accumulate microsatellite instability with acquired loss of MLH1 protein in hematopoietic stem and progenitor cells as a function of age. Blood 120:3229–3236
Keyes BE, Segal JP, Heller E, Lien WH, Chang CY, Guo X, Oristian DS, Zheng D, Fuchs E (2013) Nfatc1 orchestrates aging in hair follicle stem cells. Proc Natl Acad Sci USA 110:E4950–E4959
Kim E, Davidson LA, Zoh RS, Hensel ME, Patil BS, Jayaprakasha GK, Callaway ES, Allred CD, Turner ND, Weeks BR, Chapkin RS (2016) Homeostatic responses of colonic LGR5+ stem cells following acute in vivo exposure to a genotoxic carcinogen. Carcinogenesis 37:206–214
Kim HN, Chang J, Shao L, Han L, Iyer S, Manolagas SC, O’Brien CA, Jilka RL, Zhou D, Almeida M (2017a) DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell 16:693–703
Kim M, Rhee JK, Choi H, Kwon A, Kim J, Lee GD, Jekarl DW, Lee S, Kim Y, Kim TM (2017b) Passage-dependent accumulation of somatic mutations in mesenchymal stromal cells during in vitro culture revealed by whole genome sequencing. Sci Rep 7:14508
Kirkwood TB (1977) Evolution of ageing. Nature 270:301–304
Kong A, Frigge ML, Masson G, Besenbacher S, Sulem P, Magnusson G, Gudjonsson SA, Sigurdsson A, Jonasdottir A, Jonasdottir A, Wong WS, Sigurdsson G, Walters GB, Steinberg S, Helgason H, Thorleifsson G, Gudbjartsson DF, Helgason A, Magnusson OT, Thorsteinsdottir U, Stefansson K (2012) Rate of de novo mutations and the importance of father’s age to disease risk. Nature 488:471–475
Kornienko JS, Smirnova IS, Pugovkina NA, Ivanova JS, Shilina MA, Grinchuk TM, Shatrova AN, Aksenov ND, Zenin VV, Nikolsky NN, Lyublinskaya OG (2019) High doses of synthetic antioxidants induce premature senescence in cultivated mesenchymal stem cells. Sci Rep 9:1296
Kuhn HG, Dickinson-Anson H, Gage FH (1996) Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci 16:2027–2033
Kwon OS, Yoo HG, Han JH, Lee SR, Chung JH, Eun HC (2008) Photoaging-associated changes in epidermal proliferative cell fractions in vivo. Arch Dermatol Res 300:47–52
Lamb NE, Freeman SB, Savage-Austin A, Pettay D, Taft L, Hersey J, Gu Y, Shen J, Saker D, May KM, Avramopoulos D, Petersen MB, Hallberg A, Mikkelsen M, Hassold TJ, Sherman SL (1996) Susceptible chiasmate configurations of chromosome 21 predispose to non-disjunction in both maternal meiosis I and meiosis II. Nat Genet 14:400–405
Lamb NE, Feingold E, Savage A, Avramopoulos D, Freeman S, Gu Y, Hallberg A, Hersey J, Karadima G, Pettay D, Saker D, Shen J, Taft L, Mikkelsen M, Petersen MB, Hassold T, Sherman SL (1997) Characterization of susceptible chiasma configurations that increase the risk for maternal nondisjunction of chromosome 21. Hum Mol Genet 6:1391–1399
Latella L, Dall’Agnese A, Boscolo FS, Nardoni C, Cosentino M, Lahm A, Sacco A, Puri PL (2017) DNA damage signaling mediates the functional antagonism between replicative senescence and terminal muscle differentiation. Genes Dev 31:648–659
Lavasani M, Robinson AR, Lu A, Song M, Feduska JM, Ahani B, Tilstra JS, Feldman CH, Robbins PD, Niedernhofer LJ, Huard J (2012) Muscle-derived stem/progenitor cell dysfunction limits healthspan and lifespan in a murine progeria model. Nat Commun 3:608
Le O, Palacio L, Bernier G, Batinic-Haberle I, Hickson G, Beausejour C (2018) INK4a/ARF expression impairs neurogenesis in the brain of irradiated mice. Stem Cell Reports 10:1721–1733
Leeman DS, Hebestreit K, Ruetz T, Webb AE, McKay A, Pollina EA, Dulken BW, Zhao X, Yeo RW, Ho TT, Mahmoudi S, Devarajan K, Passegue E, Rando TA, Frydman J, Brunet A (2018) Lysosome activation clears aggregates and enhances quiescent neural stem cell activation during aging. Science 359:1277–1283
Lee-Six H, Obro NF, Shepherd MS, Grossmann S, Dawson K, Belmonte M, Osborne RJ, Huntly BJP, Martincorena I, Anderson E, O’Neill L, Stratton MR, Laurenti E, Green AR, Kent DG, Campbell PJ (2018) Population dynamics of normal human blood inferred from somatic mutations. Nature 561:473–478
Lengner CJ, Steinman HA, Gagnon J, Smith TW, Henderson JE, Kream BE, Stein GS, Lian JB, Jones SN (2006) Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling. J Cell Biol 172:909–921
L’Honore A, Commere PH, Negroni E, Pallafacchina G, Friguet B, Drouin J, Buckingham M, Montarras D (2018) The role of Pitx2 and Pitx3 in muscle stem cells gives new insights into P38alpha MAP kinase and redox regulation of muscle regeneration. Elife 7:e32991. https://doi.org/10.7554/eLife.32991.001
Liu L, Cheung TH, Charville GW, Hurgo BM, Leavitt T, Shih J, Brunet A, Rando TA (2013) Chromatin modifications as determinants of muscle stem cell quiescence and chronological aging. Cell Rep 4:189–204
Liu L, Charville GW, Cheung TH, Yoo B, Santos PJ, Schroeder M, Rando TA (2018) Impaired notch signaling leads to a decrease in p53 activity and mitotic catastrophe in aged muscle stem cells. Cell Stem Cell 23(544–56):e4
Lopez-Garcia C, Klein AM, Simons BD, Winton DJ (2010) Intestinal stem cell replacement follows a pattern of neutral drift. Science 330:822–825
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Lu Y, Song S, Jiang X, Meng Q, Wang C, Li X, Yang Y, Xin X, Zheng Q, Wang L, Pu H, Gui X, Li T, Lu D (2019) miR675 accelerates malignant transformation of mesenchymal stem cells by blocking DNA mismatch repair. Mol Ther Nucleic Acids 14:171–183
Luo S, Murphy CT (2011) Caenorhabditis elegans reproductive aging: regulation and underlying mechanisms. Genesis 49:53–65
Luo S, Kleemann GA, Ashraf JM, Shaw WM, Murphy CT (2010) TGF-beta and insulin signaling regulate reproductive aging via oocyte and germline quality maintenance. Cell 143:299–312
Ma X, Han Y, Song X, Do T, Yang Z, Ni J, Xie T (2016) DNA damage-induced Lok/CHK2 activation compromises germline stem cell self-renewal and lineage differentiation. Development 143:4312–4323
Malta TM, Sokolov A, Gentles AJ, Burzykowski T, Poisson L, Weinstein JN, Kaminska B, Huelsken J, Omberg L, Gevaert O, Colaprico A, Czerwinska P, Mazurek S, Mishra L, Heyn H, Krasnitz A, Godwin AK, Lazar AJ, Network Cancer Genome Atlas Research, Stuart JM, Hoadley KA, Laird PW, Noushmehr H, Wiznerowicz M (2018) Machine learning identifies stemness features associated with oncogenic dedifferentiation. Cell 173:338–354 (e15)
Martin K, Kirkwood TB, Potten CS (1998a) Age changes in stem cells of murine small intestinal crypts. Exp Cell Res 241:316–323
Martin K, Potten CS, Roberts SA, Kirkwood TB (1998b) Altered stem cell regeneration in irradiated intestinal crypts of senescent mice. J Cell Sci 111(Pt 16):2297–2303
Mascre G, Dekoninck S, Drogat B, Youssef KK, Brohee S, Sotiropoulou PA, Simons BD, Blanpain C (2012) Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489:257–262
Matsumura H, Mohri Y, Binh NT, Morinaga H, Fukuda M, Ito M, Kurata S, Hoeijmakers J, Nishimura EK (2016) Hair follicle aging is driven by transepidermal elimination of stem cells via COL17A1 proteolysis. Science 351:aad4395
McCay CM, Crowell MF, Maynard LA (1989) The effect of retarded growth upon the length of life span and upon the ultimate body size1935. Nutrition 5:155–171 (discussion 72)
McConnell MJ, Lindberg MR, Brennand KJ, Piper JC, Voet T, Cowing-Zitron C, Shumilina S, Lasken RS, Vermeesch JR, Hall IM, Gage FH (2013) Mosaic copy number variation in human neurons. Science 342:632–637
Mihaylova MM, Cheng CW, Cao AQ, Tripathi S, Mana MD, Bauer-Rowe KE, Abu-Remaileh M, Clavain L, Erdemir A, Lewis CA, Freinkman E, Dickey AS, La Spada AR, Huang Y, Bell GW, Deshpande V, Carmeliet P, Katajisto P, Sabatini DM, Yilmaz OH (2018) Fasting activates fatty acid oxidation to enhance intestinal stem cell function during homeostasis and aging. Cell Stem Cell 22:769–778 (e4)
Mineyeva OA, Bezriadnov DV, Kedrov AV, Lazutkin AA, Anokhin KV, Enikolopov GN (2018) Radiation induces distinct changes in defined subpopulations of neural stem and progenitor cells in the adult hippocampus. Front Neurosci 12:1013
Miranda JP, Camoes SP, Gaspar MM, Rodrigues JS, Carvalheiro M, Barcia RN, Cruz P, Cruz H, Simoes S, Santos JM (2019) The secretome derived from 3D-cultured umbilical cord tissue MSCs counteracts manifestations typifying rheumatoid arthritis. Front Immunol 10:18
Moehrle BM, Nattamai K, Brown A, Florian MC, Ryan M, Vogel M, Bliederhaeuser C, Soller K, Prows DR, Abdollahi A, Schleimer D, Walter D, Milsom MD, Stambrook P, Porteus M, Geiger H (2015) Stem cell-specific mechanisms ensure genomic fidelity within HSCs and upon aging of HSCs. Cell Rep 13:2412–2424
Moorefield EC, Andres SF, Blue RE, Van Landeghem L, Mah AT, Santoro MA, Ding S (2017) Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging (Albany NY) 9:1898–1915
Moskalev AA, Shaposhnikov MV, Plyusnina EN, Zhavoronkov A, Budovsky A, Yanai H, Fraifeld VE (2013) The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res Rev 12:661–684
Murga M, Bunting S, Montana MF, Soria R, Mulero F, Canamero M, Lee Y, McKinnon PJ, Nussenzweig A, Fernandez-Capetillo O (2009) A mouse model of ATR-Seckel shows embryonic replicative stress and accelerated aging. Nat Genet 41:891–898
Myant KB, Cammareri P, Hodder MC, Wills J, Von Kriegsheim A, Gyorffy B, Rashid M, Polo S, Maspero E, Vaughan L, Gurung B, Barry E, Malliri A, Camargo F, Adams DJ, Iavarone A, Lasorella A, Sansom OJ (2017) ‘HUWE1 is a critical colonic tumour suppressor gene that prevents MYC signalling, DNA damage accumulation and tumour initiation. EMBO Mol Med 9:181–197
Na HJ, Park JS, Pyo JH, Lee SH, Jeon HJ, Kim YS, Yoo MA (2013) Mechanism of metformin: inhibition of DNA damage and proliferative activity in Drosophila midgut stem cell. Mech Ageing Dev 134:381–390
Nagaoka SI, Hassold TJ, Hunt PA (2012) Human aneuploidy: mechanisms and new insights into an age-old problem. Nat Rev Genet 13:493–504
Nagy P, Sandor GO, Juhasz G (2018) Autophagy maintains stem cells and intestinal homeostasis in Drosophila. Sci Rep 8:4644
Nalapareddy K, Nattamai KJ, Kumar RS, Karns R, Wikenheiser-Brokamp KA, Sampson LL, Mahe MM, Sundaram N, Yacyshyn MB, Yacyshyn B, Helmrath MA, Zheng Y, Geiger H (2017) Canonical Wnt signaling ameliorates aging of intestinal stem cells. Cell Rep 18:2608–2621
Nicolaije C, Diderich KE, Botter SM, Priemel M, Waarsing JH, Day JS, Brandt RM, Schilling AF, Weinans H, Van der Eerden BC, van der Horst GT, Hoeijmakers JH, van Leeuwen JP (2012) Age-related skeletal dynamics and decrease in bone strength in DNA repair deficient male trichothiodystrophy mice. PLoS One 7:e35246
Niedernhofer LJ, Garinis GA, Raams A, Lalai AS, Robinson AR, Appeldoorn E, Odijk H, Oostendorp R, Ahmad A, van Leeuwen W, Theil AF, Vermeulen W, van der Horst GT, Meinecke P, Kleijer WJ, Vijg J, Jaspers NG, Hoeijmakers JH (2006) A new progeroid syndrome reveals that genotoxic stress suppresses the somatotroph axis. Nature 444:1038–1043
Nijnik A, Woodbine L, Marchetti C, Dawson S, Lambe T, Liu C, Rodrigues NP, Crockford TL, Cabuy E, Vindigni A, Enver T, Bell JI, Slijepcevic P, Goodnow CC, Jeggo PA, Cornall RJ (2007) DNA repair is limiting for haematopoietic stem cells during ageing. Nature 447:686–690
Nishino J, Kim I, Chada K, Morrison SJ (2008) Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf expression. Cell 135:227–239
Nokia MS, Lensu S, Ahtiainen JP, Johansson PP, Koch LG, Britton SL, Kainulainen H (2016) Physical exercise increases adult hippocampal neurogenesis in male rats provided it is aerobic and sustained. J Physiol 594:1855–1873
Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, Silberstein LE (2013) Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 15:533–543
Norddahl GL, Pronk CJ, Wahlestedt M, Sten G, Nygren JM, Ugale A, Sigvardsson M, Bryder D (2011) Accumulating mitochondrial DNA mutations drive premature hematopoietic aging phenotypes distinct from physiological stem cell aging. Cell Stem Cell 8:499–510
Ogasawara Y, Nakayama K, Tarnowka M, McCoy JP Jr, Kajigaya S, Levin BC, Young NS (2005) Mitochondrial DNA spectra of single human CD34 + cells, T cells, B cells, and granulocytes. Blood 106:3271–3284
Oliver TR, Feingold E, Yu K, Cheung V, Tinker S, Yadav-Shah M, Masse N, Sherman SL (2008) New insights into human nondisjunction of chromosome 21 in oocytes. PLoS Genet 4:e1000033
Osorio FG, Rosendahl Huber A, Oka R, Verheul M, Patel SH, Hasaart K, de la Fonteijne L, Varela I, Camargo FD, van Boxtel R (2018) Somatic mutations reveal lineage relationships and age-related mutagenesis in human hematopoiesis. Cell Rep 25(2308–16):e4
Otsuka K, Suzuki K, Fujimichi Y, Tomita M, Iwasaki T (2018) Cellular responses and gene expression profiles of colonic Lgr5 + stem cells after low-dose/low-dose-rate radiation exposure. J Radiat Res 59:ii8–ii22
Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL (2011) Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age. Proc Natl Acad Sci USA 108:20012–20017
Panich U, Sittithumcharee G, Rathviboon N, Jirawatnotai S (2016) Ultraviolet radiation-induced skin aging: the role of DNA damage and oxidative stress in epidermal stem cell damage mediated skin aging. Stem Cells Int 2016:7370642
Park JS, Lee SH, Na HJ, Pyo JH, Kim YS, Yoo MA (2012) Age- and oxidative stress-induced DNA damage in Drosophila intestinal stem cells as marked by Gamma-H2AX. Exp Gerontol 47:401–405
Park JS, Pyo JH, Na HJ, Jeon HJ, Kim YS, Arking R, Yoo MA (2014) Increased centrosome amplification in aged stem cells of the Drosophila midgut. Biochem Biophys Res Commun 450:961–965
Park JS, Na HJ, Pyo JH, Jeon HJ, Kim YS, Yoo MA (2015) Requirement of ATR for maintenance of intestinal stem cells in aging Drosophila. Aging (Albany NY) 7:307–318
Park JS, Jeon HJ, Pyo JH, Kim YS, Yoo MA (2018) Deficiency in DNA damage response of enterocytes accelerates intestinal stem cell aging in Drosophila. Aging (Albany NY) 10:322–338
Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD (2007) Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA 104:5431–5436
Paul C, Nagano M, Robaire B (2011) Aging results in differential regulation of DNA repair pathways in pachytene spermatocytes in the Brown Norway rat. Biol Reprod 85:1269–1278
Paul C, Nagano M, Robaire B (2013) Aging results in molecular changes in an enriched population of undifferentiated rat spermatogonia. Biol Reprod 89:147
Pech MF, Garbuzov A, Hasegawa K, Sukhwani M, Zhang RJ, Benayoun BA, Brockman SA, Lin S, Brunet A, Orwig KE, Artandi SE (2015) High telomerase is a hallmark of undifferentiated spermatogonia and is required for maintenance of male germline stem cells. Genes Dev 29:2420–2434
Pilzecker B, Buoninfante OA, van den Berk P, Lancini C, Song JY, Citterio E, Jacobs H (2017) DNA damage tolerance in hematopoietic stem and progenitor cells in mice. Proc Natl Acad Sci USA 114:E6875–E6883
Pino AM, Rosen CJ, Rodriguez JP (2012) In osteoporosis, differentiation of mesenchymal stem cells (MSCs) improves bone marrow adipogenesis. Biol Res 45:279–287
Pinto M, Pickrell AM, Wang X, Bacman SR, Yu A, Hida A, Dillon LM, Morton PD, Malek TR, Williams SL, Moraes CT (2017) Transient mitochondrial DNA double strand breaks in mice cause accelerated aging phenotypes in a ROS-dependent but p53/p21-independent manner. Cell Death Differ 24:288–299
Porto ML, Rodrigues BP, Menezes TN, Ceschim SL, Casarini DE, Gava AL, Pereira TM, Vasquez EC, Campagnaro BP, Meyrelles SS (2015) Reactive oxygen species contribute to dysfunction of bone marrow hematopoietic stem cells in aged C57BL/6 J mice. J Biomed Sci 22:97
Prasher JM, Lalai AS, Heijmans-Antonissen C, Ploemacher RE, Hoeijmakers JH, Touw IP, Niedernhofer LJ (2005) Reduced hematopoietic reserves in DNA interstrand crosslink repair-deficient Ercc1−/− mice. EMBO J 24:861–871
Pugh JL, Foster SA, Sukhina AS, Petravic J, Uhrlaub JL, Padilla-Torres J, Hayashi T, Nakachi K, Smithey MJ, Nikolich-Zugich J (2016) Acute systemic DNA damage in youth does not impair immune defense with aging. Aging Cell 15:686–693
Qiao H, Hbdp Rao Y, Yun S, Sandhu JH, Fong M, Sapre M, Nguyen A, Van Tham BW, Chng TYH, Lee A, Hunter N (2018) Impeding DNA break repair enables oocyte quality control. mol cell 72(211–21):e3
Qing Y, Gerson SL (2017) Mismatch repair deficient hematopoietic stem cells are preleukemic stem cells. PLoS One 12:e0182175
Rasheed N, Wang X, Niu QT, Yeh J, Li B (2006) Atm-deficient mice: an osteoporosis model with defective osteoblast differentiation and increased osteoclastogenesis. Hum Mol Genet 15:1938–1948
Ray S, Corenblum MJ, Anandhan A, Reed A, Ortiz FO, Zhang DD, Barnes CA, Madhavan L (2018) A Role for Nrf2 expression in defining the aging of hippocampal neural stem cells. Cell Transplant 27:589–606
Rinaldi VD, Bolcun-Filas E, Kogo H, Kurahashi H, Schimenti JC (2017) The DNA damage checkpoint eliminates mouse oocytes with chromosome synapsis failure. Mol Cell 67(1026–36):e2
Risch N, Reich EW, Wishnick MM, McCarthy JG (1987) Spontaneous mutation and parental age in humans. Am J Hum Genet 41:218–248
Romano FJ, Rossetti S, Conteduca V, Schepisi G, Cavaliere C, Di Franco R, La Mantia E, Castaldo L, Nocerino F, Ametrano G, Cappuccio F, Malzone G, Montanari M, Vanacore D, Quagliariello V, Piscitelli R, Pepe MF, Berretta M, D’Aniello C, Perdona S, Muto P, Botti G, Ciliberto G, Veneziani BM, De Falco F, Maiolino P, Caraglia M, Montella M, De Giorgi U, Facchini G (2016) Role of DNA repair machinery and p53 in the testicular germ cell cancer: a review. Oncotarget 7:85641–85649
Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL (2007) Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature 447:725–729
Rouanet S, Warrick E, Gache Y, Scarzello S, Avril MF, Bernerd F, Magnaldo T (2013) Genetic correction of stem cells in the treatment of inherited diseases and focus on xeroderma pigmentosum. Int J Mol Sci 14:20019–20036
Rube CE, Fricke A, Widmann TA, Furst T, Madry H, Pfreundschuh M, Rube C (2011a) Accumulation of DNA damage in hematopoietic stem and progenitor cells during human aging. PLoS One 6:e17487
Rube CE, Zhang S, Miebach N, Fricke A, Rube C (2011b) Protecting the heritable genome: dNA damage response mechanisms in spermatogonial stem cells. DNA Repair (Amst) 10:159–168
Ruetze M, Dunckelmann K, Schade A, Reuschlein K, Mielke H, Weise JM, Gallinat S, Wenck H, Knott A (2011) Damage at the root of cell renewal–UV sensitivity of human epidermal stem cells. J Dermatol Sci 64:16–22
Ruzankina Y, Pinzon-Guzman C, Asare A, Ong T, Pontano L, Cotsarelis G, Zediak VP, Velez M, Bhandoola A, Brown EJ (2007) Deletion of the developmentally essential gene ATR in adult mice leads to age-related phenotypes and stem cell loss. Cell Stem Cell 1:113–126
Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL (2006) Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells 24:1505–1511
Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM (2008) Self-renewal and expansion of single transplanted muscle stem cells. Nature 456:502–506
Salk JJ, Fox EJ, Loeb LA (2010) Mutational heterogeneity in human cancers: origin and consequences. Annu Rev Pathol 5:51–75
Samper E, Fernandez P, Eguia R, Martin-Rivera L, Bernad A, Blasco MA, Aracil M (2002) Long-term repopulating ability of telomerase-deficient murine hematopoietic stem cells. Blood 99:2767–2775
Sanchez-Danes A, Hannezo E, Larsimont JC, Liagre M, Youssef KK, Simons BD, Blanpain C (2016) Defining the clonal dynamics leading to mouse skin tumour initiation. Nature 536:298–303
Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, Anderson D, Wyrobek AJ (2007) The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod 22:180–187
Schmid TE, Grant PG, Marchetti F, Weldon RH, Eskenazi B, Wyrobek AJ (2013) Elemental composition of human semen is associated with motility and genomic sperm defects among older men. Hum Reprod 28:274–282
Schneider L, Pellegatta S, Favaro R, Pisati F, Roncaglia P, Testa G, Nicolis SK, Finocchiaro G, d’Adda di Fagagna F (2013) DNA damage in mammalian neural stem cells leads to astrocytic differentiation mediated by BMP2 signaling through JAK-STAT. Stem Cell Reports 1:123–138
Schuler N, Rube CE (2013) Accumulation of DNA damage-induced chromatin alterations in tissue-specific stem cells: the driving force of aging? PLoS One 8:e63932
Schwer B, Wei PC, Chang AN, Kao J, Du Z, Meyers RM, Alt FW (2016) Transcription-associated processes cause DNA double-strand breaks and translocations in neural stem/progenitor cells. Proc Natl Acad Sci USA 113:2258–2263
Schworer S, Becker F, Feller C, Baig AH, Kober U, Henze H, Kraus JM, Xin B, Lechel A, Lipka DB, Varghese CS, Schmidt M, Rohs R, Aebersold R, Medina KL, Kestler HA, Neri F, von Maltzahn J, Tumpel S, Rudolph KL (2016) Epigenetic stress responses induce muscle stem-cell ageing by Hoxa9 developmental signals. Nature 540:428–432
Selesniemi K, Lee HJ, Muhlhauser A, Tilly JL (2011) Prevention of maternal aging-associated oocyte aneuploidy and meiotic spindle defects in mice by dietary and genetic strategies. Proc Natl Acad Sci USA 108:12319–12324
Sethe S, Scutt A, Stolzing A (2006) Aging of mesenchymal stem cells. Ageing Res Rev 5:91–116
Sharma N, Colangelo NW, de Toledo SM, Azzam EI (2016) Diffusible factors secreted by glioblastoma and medulloblastoma cells induce oxidative stress in bystander neural stem progenitors. ASN Neuro 8(4):1759091416662808. https://doi.org/10.1177/1759091416662808
Shin MG, Kajigaya S, McCoy JP Jr, Levin BC, Young NS (2004) Marked mitochondrial DNA sequence heterogeneity in single CD34 + cell clones from normal adult bone marrow. Blood 103:553–561
Shlush LI, Zandi S, Mitchell A, Chen WC, Brandwein JM, Gupta V, Kennedy JA, Schimmer AD, Schuh AC, Yee KW, McLeod JL, Doedens M, Medeiros JJ, Marke R, Kim HJ, Lee K, McPherson JD, Hudson TJ, Halt Pan-Leukemia Gene Panel Consortium, Brown AM, Yousif F, Trinh QM, Stein LD, Minden MD, Wang JC, Dick JE (2014) Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 506:328–333
Shook BA, Manz DH, Peters JJ, Kang S, Conover JC (2012) Spatiotemporal changes to the subventricular zone stem cell pool through aging. J Neurosci 32:6947–6956
Short NJ, Rytting ME, Cortes JE (2018) Acute myeloid leukaemia. Lancet 392:593–606
Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, Sadek HA (2010) The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7:380–390
Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ (2014) Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science 344:649–652
Siudeja K, Nassari S, Gervais L, Skorski P, Lameiras S, Stolfa D, Zande M, Bernard V, Frio TR, Bardin AJ (2015) Frequent somatic mutation in adult intestinal stem cells drives neoplasia and genetic mosaicism during aging. Cell Stem Cell 17:663–674
Sloter ED, Marchetti F, Eskenazi B, Weldon RH, Nath J, Cabreros D, Wyrobek AJ (2007) Frequency of human sperm carrying structural aberrations of chromosome 1 increases with advancing age. Fertil Steril 87:1077–1086
Snippert HJ, Schepers AG, van Es JH, Simons BD, Clevers H (2014) Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion. EMBO Rep 15:62–69
Solanas G, Peixoto FO, Perdiguero E, Jardi M, Ruiz-Bonilla V, Datta D, Symeonidi A, Castellanos A, Welz PS, Caballero JM, Sassone-Corsi P, Munoz-Canoves P, Benitah SA (2017) Aged stem cells reprogram their daily rhythmic functions to adapt to stress. Cell 170(678–92):e20
Sotiropoulou PA, Blanpain C (2012) Development and homeostasis of the skin epidermis. Cold Spring Harb Perspect Biol 4:a008383
Sotiropoulou PA, Karambelas AE, Debaugnies M, Candi A, Bouwman P, Moers V, Revenco T, Rocha AS, Sekiguchi K, Jonkers J, Blanpain C (2013) BRCA1 deficiency in skin epidermis leads to selective loss of hair follicle stem cells and their progeny. Genes Dev 27:39–51
Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, Jardi M, Ballestar E, Gonzalez S, Serrano AL, Perdiguero E, Munoz-Canoves P (2014) Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 506:316–321
Sousa-Victor P, Ayyaz A, Hayashi R, Qi Y, Madden DT, Lunyak VV, Jasper H (2017) Piwi is required to limit exhaustion of aging somatic stem cells. Cell Rep 20:2527–2537
Steuerwald NM, Bermudez MG, Wells D, Munne S, Cohen J (2007) Maternal age-related differential global expression profiles observed in human oocytes. Reprod Biomed Online 14:700–708
Stringer JM, Winship A, Liew SH, Hutt K (2018) The capacity of oocytes for DNA repair. Cell Mol Life Sci 75:2777–2792
Stucker M, Struk A, Altmeyer P, Herde M, Baumgartl H, Lubbers DW (2002) The cutaneous uptake of atmospheric oxygen contributes significantly to the oxygen supply of human dermis and epidermis. J Physiol 538:985–994
Su X, Paris M, Gi YJ, Tsai KY, Cho MS, Lin YL, Biernaskie JA, Sinha S, Prives C, Pevny LH, Miller FD, Flores ER (2009) TAp63 prevents premature aging by promoting adult stem cell maintenance. Cell Stem Cell 5:64–75
Suda T, Takubo K, Semenza GL (2011) Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9:298–310
Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, Wang H, Le T, Faull KF, Chen R, Gu H, Bock C, Meissner A, Gottgens B, Darlington GJ, Li W, Goodell MA (2014) Epigenomic profiling of young and aged hscs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 14:673–688
Szilard L (1959) On the nature of the aging process. Proc Natl Acad Sci USA 45:30–45
Takayama K, Kawakami Y, Lavasani M, Mu X, Cummins JH, Yurube T, Kuroda R, Kurosaka M, Fu FH, Robbins PD, Niedernhofer LJ, Huard J (2017) mTOR signaling plays a critical role in the defects observed in muscle-derived stem/progenitor cells isolated from a murine model of accelerated aging. J Orthop Res 35:1375–1382
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA (2011) Interconversion between intestinal stem cell populations in distinct niches. Science 334:1420–1424
Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T (2010) Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7:391–402
Tang L, Bergevoet SM, Gilissen C, de Witte T, Jansen JH, van der Reijden BA, Raymakers RA (2010) Hematopoietic stem cells exhibit a specific ABC transporter gene expression profile clearly distinct from other stem cells. BMC Pharmacol 10:12
Taylor RW, Barron MJ, Borthwick GM, Gospel A, Chinnery PF, Samuels DC, Taylor GA, Plusa SM, Needham SJ, Greaves LC, Kirkwood TB, Turnbull DM (2003) Mitochondrial DNA mutations in human colonic crypt stem cells. J Clin Invest 112:1351–1360
Templado C, Donate A, Giraldo J, Bosch M, Estop A (2011) Advanced age increases chromosome structural abnormalities in human spermatozoa. Eur J Hum Genet 19:145–151
Testa U, Pelosi E, Castelli G (2018) Colorectal cancer: genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel) 6(2):31. https://doi.org/10.3390/medsci6020031
Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, de Sauvage FJ (2011) A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature 478:255–259
Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K (2013) Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med 5:172ra21
Tomasetti C, Vogelstein B (2015) Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347:78–81
Tumbar T, Guasch G, Greco V, Blanpain C, Lowry WE, Rendl M, Fuchs E (2004) Defining the epithelial stem cell niche in skin. Science 303:359–363
Tuppi M, Kehrloesser S, Coutandin DW, Rossi V, Luh LM, Strubel A, Hotte K, Hoffmeister M, Schafer B, De Oliveira T, Greten F, Stelzer EHK, Knapp S, De Felici M, Behrends C, Klinger FG, Dotsch V (2018) Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol 25:261–269
van der Heijden M, Zimberlin CD, Nicholson AM, Colak S, Kemp R, Meijer SL, Medema JP, Greten FR, Jansen M, Winton DJ, Vermeulen L (2016) Bcl-2 is a critical mediator of intestinal transformation. Nat Commun 7:10916
Vina J, Borras C, Miquel J (2007) Theories of ageing. IUBMB Life 59:249–254
Wagers AJ, Weissman IL (2004) Plasticity of adult stem cells. Cell 116:639–648
Walkley CR, Qudsi R, Sankaran VG, Perry JA, Gostissa M, Roth SI, Rodda SJ, Snay E, Dunning P, Fahey FH, Alt FW, McMahon AP, Orkin SH (2008) Conditional mouse osteosarcoma, dependent on p53 loss and potentiated by loss of Rb, mimics the human disease. Genes Dev 22:1662–1676
Wang X, Kua HY, Hu Y, Guo K, Zeng Q, Wu Q, Ng HH, Karsenty G, de Crombrugghe B, Yeh J, Li B (2006) p53 functions as a negative regulator of osteoblastogenesis, osteoblast-dependent osteoclastogenesis, and bone remodeling. J Cell Biol 172:115–125
Wang H, Chen Q, Lee SH, Choi Y, Johnson FB, Pignolo RJ (2012a) Impairment of osteoblast differentiation due to proliferation-independent telomere dysfunction in mouse models of accelerated aging. Aging Cell 11:704–713
Wang J, Sun Q, Morita Y, Jiang H, Gross A, Lechel A, Hildner K, Guachalla LM, Gompf A, Hartmann D, Schambach A, Wuestefeld T, Dauch D, Schrezenmeier H, Hofmann WK, Nakauchi H, Ju Z, Kestler HA, Zender L, Rudolph KL (2012b) A differentiation checkpoint limits hematopoietic stem cell self-renewal in response to DNA damage. Cell 148:1001–1014
Wang J, Lu X, Sakk V, Klein CA, Rudolph KL (2014) Senescence and apoptosis block hematopoietic activation of quiescent hematopoietic stem cells with short telomeres. Blood 124:3237–3240
Wang C, Oshima M, Sashida G, Tomioka T, Hasegawa N, Mochizuki-Kashio M, Nakajima-Takagi Y, Kusunoki Y, Kyoizumi S, Imai K, Nakachi K, Iwama A (2015) Non-lethal ionizing radiation promotes aging-like phenotypic changes of human hematopoietic stem and progenitor cells in humanized mice. PLoS One 10:e0132041
Wei PC, Chang AN, Kao J, Du Z, Meyers RM, Alt FW, Schwer B (2016) Long neural genes harbor recurrent DNA break clusters in neural stem/progenitor cells. Cell 164:644–655
Wei PC, Lee CS, Du Z, Schwer B, Zhang Y, Kao J, Zurita J, Alt FW (2018) Three classes of recurrent DNA break clusters in brain progenitors identified by 3D proximity-based break joining assay. Proc Natl Acad Sci USA 115:1919–1924
Weissman IL, Shizuru JA (2008) The origins of the identification and isolation of hematopoietic stem cells, and their capability to induce donor-specific transplantation tolerance and treat autoimmune diseases. Blood 112:3543–3553
Welch JS, Ley TJ, Link DC, Miller CA, Larson DE, Koboldt DC, Wartman LD, Lamprecht TL, Liu F, Xia J, Kandoth C, Fulton RS, McLellan MD, Dooling DJ, Wallis JW, Chen K, Harris CC, Schmidt HK, Kalicki-Veizer JM, Lu C, Zhang Q, Lin L, O’Laughlin MD, McMichael JF, Delehaunty KD, Fulton LA, Magrini VJ, McGrath SD, Demeter RT, Vickery TL, Hundal J, Cook LL, Swift GW, Reed JP, Alldredge PA, Wylie TN, Walker JR, Watson MA, Heath SE, Shannon WD, Varghese N, Nagarajan R, Payton JE, Baty JD, Kulkarni S, Klco JM, Tomasson MH, Westervelt P, Walter MJ, Graubert TA, DiPersio JF, Ding L, Mardis ER, Wilson RK (2012) The origin and evolution of mutations in acute myeloid leukemia. Cell 150:264–278
Williams GC (1957) Pleiotropy, natural-selection, and the evolution of senescence. Evolution 11:398–411
Wilson A, Laurenti E, Oser G, van der Wath RC, Blanco-Bose W, Jaworski M, Offner S, Dunant CF, Eshkind L, Bockamp E, Lio P, Macdonald HR, Trumpp A (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135:1118–1129
Wingert S, Thalheimer FB, Haetscher N, Rehage M, Schroeder T, Rieger MA (2016) DNA-damage response gene GADD45A induces differentiation in hematopoietic stem cells without inhibiting cell cycle or survival. Stem Cells 34:699–710
Wong KK, Maser RS, Bachoo RM, Menon J, Carrasco DR, Gu Y, Alt FW, DePinho RA (2003) Telomere dysfunction and Atm deficiency compromises organ homeostasis and accelerates ageing. Nature 421:643–648
Wu PK, Wang JY, Chen CF, Chao KY, Chang MC, Chen WM, Hung SC (2017) Early passage mesenchymal stem cells display decreased radiosensitivity and increased DNA repair activity. Stem Cells Transl Med 6:1504–1514
Wu Z, Zhang W, Song M, Wang W, Wei G, Li W, Lei J, Huang Y, Sang Y, Chan P, Chen C, Qu J, Suzuki K, Belmonte JCI, Liu GH (2018) Differential stem cell aging kinetics in Hutchinson–Gilford progeria syndrome and Werner syndrome. Protein Cell 9:333–350
Wyrobek AJ, Eskenazi B, Young S, Arnheim N, Tiemann-Boege I, Jabs EW, Glaser RL, Pearson FS, Evenson D (2006) Advancing age has differential effects on DNA damage, chromatin integrity, gene mutations, and aneuploidies in sperm. Proc Natl Acad Sci USA 103:9601–9606
Xia J, Han L, Zhao Z (2012) Investigating the relationship of DNA methylation with mutation rate and allele frequency in the human genome. BMC Genomics 13(Suppl 8):S7
Yan CT, Kaushal D, Murphy M, Zhang Y, Datta A, Chen C, Monroe B, Mostoslavsky G, Coakley K, Gao Y, Mills KD, Fazeli AP, Tepsuporn S, Hall G, Mulligan R, Fox E, Bronson R, De Girolami U, Lee C, Alt FW (2006) XRCC4 suppresses medulloblastomas with recurrent translocations in p53-deficient mice. Proc Natl Acad Sci USA 103:7378–7383
Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, Su N, Luo Y, Heilshorn SC, Amieva MR, Sangiorgi E, Capecchi MR, Kuo CJ (2012) The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci USA 109:466–471
Yao YG, Kajigaya S, Feng X, Samsel L, McCoy JP Jr, Torelli G, Young NS (2013) Accumulation of mtDNA variations in human single CD34 + cells from maternally related individuals: effects of aging and family genetic background. Stem Cell Res 10:361–370
Yousefi M, Nakauka-Ddamba A, Berry CT, Li N, Schoenberger J, Simeonov KP, Cedeno RJ, Yu Z, Lengner CJ (2018) Calorie restriction governs intestinal epithelial regeneration through cell-autonomous regulation of mTORC1 in reserve stem cells. Stem Cell Rep 10:703–711
Yu J, Shi J, Zhang Y, Zhang Y, Huang Y, Chen Z, Yang J (2018) The replicative senescent mesenchymal stem/stromal cells defect in DNA damage response and anti-oxidative capacity. Int J Med Sci 15:771–781
Zhang X, Ebata KT, Robaire B, Nagano MC (2006) Aging of male germ line stem cells in mice. Biol Reprod 74:119–124
Zhang DY, Wang HJ, Tan YZ (2011a) Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through the DNA damage response and the p53/p21 pathway. PLoS One 6:e21397
Zhang J, Lian Q, Zhu G, Zhou F, Sui L, Tan C, Mutalif RA, Navasankari R, Zhang Y, Tse HF, Stewart CL, Colman A (2011b) A human iPSC model of Hutchinson Gilford progeria reveals vascular smooth muscle and mesenchymal stem cell defects. Cell Stem Cell 8:31–45
Zhang DY, Pan Y, Zhang C, Yan BX, Yu SS, Wu DL, Shi MM, Shi K, Cai XX, Zhou SS, Wang JB, Pan JP, Zhang LH (2013) Wnt/beta-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem 374:13–20
Zhang W, Li J, Suzuki K, Qu J, Wang P, Zhou J, Liu X, Ren R, Xu X, Ocampo A, Yuan T, Yang J, Li Y, Shi L, Guan D, Pan H, Duan S, Ding Z, Li M, Yi F, Bai R, Wang Y, Chen C, Yang F, Li X, Wang Z, Aizawa E, Goebl A, Soligalla RD, Reddy P, Esteban CR, Tang F, Liu GH, Belmonte JC (2015) Aging stem cells. A Werner syndrome stem cell model unveils heterochromatin alterations as a driver of human aging. Science 348:1160–1163
Zhao R, Xuan Y, Li X, Xi R (2008) Age-related changes of germline stem cell activity, niche signaling activity and egg production in Drosophila. Aging Cell 7:344–354
Zhou S, Morris JJ, Barnes Y, Lan L, Schuetz JD, Sorrentino BP (2002) Bcrp1 gene expression is required for normal numbers of side population stem cells in mice, and confers relative protection to mitoxantrone in hematopoietic cells in vivo. Proc Natl Acad Sci USA 99:12339–12344
Zhou T, Hasty P, Walter CA, Bishop AJ, Scott LM, Rebel VI (2013) Myelodysplastic syndrome: an inability to appropriately respond to damaged DNA? Exp Hematol 41:665–674
Zhou T, Chen P, Gu J, Bishop AJ, Scott LM, Hasty P, Rebel VI (2015) Potential relationship between inadequate response to DNA damage and development of myelodysplastic syndrome. Int J Mol Sci 16:966–989
Acknowledgements
This research was supported by the Intramural Research Program of the National Institute on Aging, National Institutes of Health. We apologize for the inability to cite all relevant manuscripts.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McNeely, T., Leone, M., Yanai, H. et al. DNA damage in aging, the stem cell perspective. Hum Genet 139, 309–331 (2020). https://doi.org/10.1007/s00439-019-02047-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-019-02047-z