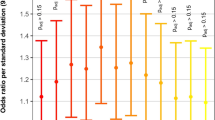
Abstract
Congenital Diaphragmatic Hernia (CDH) is a common and often lethal birth defect characterized by diaphragmatic structural defects and pulmonary hypoplasia. CDH is isolated in 60% of newborns, but may also be part of a complex phenotype with additional anomalies. We performed whole exome sequencing (WES) on 87 individuals with isolated or complex CDH and on their unaffected parents, to assess the contribution of de novo mutations in the etiology of diaphragmatic and pulmonary defects and to identify new candidate genes. A combined analysis with 39 additional trios with complex CDH, previously published, revealed a significant genome-wide burden of de novo variants compared to background mutation rate and 900 control trios. We identified an increased burden of likely gene-disrupting (LGD, i.e. nonsense, frameshift, and canonical splice site) and predicted deleterious missense (D-mis) variants in complex and isolated CDH patients. Overall, an excess of predicted damaging de novo LGD and D-mis variants relative to the expected frequency contributed to 21% of complex cases and 12% of isolated CDH cases. The burden of de novo variants was higher in genes expressed in the developing mouse diaphragm and heart. Some overlap with genes responsible for congenital heart defects and neurodevelopmental disorders was observed in CDH patients within our cohorts. We propose that de novo variants contribute significantly to the development of CDH.




Similar content being viewed by others
References
Ackerman KG, Vargas SO, Wilson JA et al (2012) Congenital diaphragmatic defects: proposal for a new classification based on observations in 234 patients. Pediatr Dev Pathol 15(4):265–274. doi:10.2350/11-05-1041-OA.1
Beauchemin KJ, Wells JM, Kho AT et al (2016) Temporal dynamics of the developing lung transcriptome in three common inbred strains of laboratory mice reveals multiple stages of postnatal alveolar development. PeerJ 4:e2318. doi:10.7717/peerj.2318
Borensztein M, Monnier P, Court F et al (2013) Myod and H19-Igf2 locus interactions are required for diaphragm formation in the mouse. Development 140(6):1231–1239. doi:10.1242/dev.084665
Cao XR, Lill NL, Boase N et al (2008) Nedd4 controls animal growth by regulating IGF-1 signaling. Sci Signal 1(38):ra5. doi:10.1126/scisignal.1160940
Chen EY, Tan CM, Kou Y et al (2013) Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics 14:128. doi:10.1186/1471-2105-14-128
De Rubeis S, He X, Goldberg AP et al (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515:209–215. doi:10.1038/nature13772
Donahoe PK, Longoni M, High FA (2016) Polygenic causes of congenital diaphragmatic hernia produce common lung pathologies: a multimodal war on congenital diaphragmatic hernia. Am J Pathol. doi:10.1016/j.ajpath.2016.07.006
Dong C, Wei P, Jian X et al (2015) Comparison and integration of deleteriousness prediction methods for nonsynonymous SNVs in whole exome sequencing studies. Hum Mol Genet 24:2125–2137. doi:10.1093/hmg/ddu733
Epaud R, Aubey F, Xu J et al (2012) Knockout of insulin-like growth factor-1 receptor impairs distal lung morphogenesis. PLoS One 7(11):e48071. doi:10.1371/journal.pone.0048071
Garcia AV, Thirumoorthi AS, Stolar CJH (2014) Extracorporeal membrane oxygenation. In: Saunders L (ed) Ashcraft’s Pediatric Surgery, 6th edn. Saunders, London, pp 80–93
Gulsuner S, Walsh T, Watts AC et al (2013) Spatial and temporal mapping of de novo mutations in schizophrenia to a fetal prefrontal cortical network. Cell 154:518–529. doi:10.1016/j.cell.2013.06.049
Homsy J, Zaidi S, Shen Y et al (2015) De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science 350:1262–1266. doi:10.1126/science.aac9396
Iossifov I, O’roak BJ, Sanders SJ et al (2014) The contribution of de novo coding mutations to autism spectrum disorder. 216–221. doi:10.15154/1149697
Keijzer R, Liu J, Deimling J et al (2000) Dual-hit hypothesis explains pulmonary hypoplasia in the nitrofen model of congenital diaphragmatic hernia. Am J Pathol 156(4):1299–1306
Kinane TB (2007) Lung development and implications for hypoplasia found in congenital diaphragmatic hernia. Am J Med Genet C Semin Med Genet. 145C(2):117–124
Lachmann A, Xu H, Krishnan J et al (2010) ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics 26:2438–2444. doi:10.1093/bioinformatics/btq466
Lage K, Karlberg EO, Størling ZM et al (2007) A human phenome-interactome network of protein complexes implicated in genetic disorders. Nat Biotechnol 25:309–316. doi:10.1038/nbt1295
Langfelder P, Horvath S (2008) WGCNA: an R package for weighted correlation network analysis. BMC Bioinform 9:559. doi:10.1186/1471-2105-9-559
Lek M, Karczewski KJ, Minikel EV et al (2016) Analysis of protein-coding genetic variation in 60,706 humans. Nature 536(7616):285–291. doi:10.1038/nature19057
Li H, Durbin R (2010) Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26:589–595. doi:10.1093/bioinformatics/btp698
Li H, Handsaker B, Wysoker A et al (2009) The sequence alignment/Map format and SAMtools. Bioinformatics 25:2078–2079. doi:10.1093/bioinformatics/btp352
Liu JP, Baker J, Perkins AS et al (1993) Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 75(1):59–72
Longoni M, High FA, Russell MK et al (2014a) Molecular pathogenesis of congenital diaphragmatic hernia revealed by exome sequencing, developmental data, and bioinformatics. Proc Natl Acad Sci USA 111:12450–12455. doi:10.1073/pnas.1412509111
Longoni M, Russell MK, High FA et al (2014b) Prevalence and penetrance of ZFPM2 mutations and deletions causing congenital diaphragmatic hernia. Clin Genet. doi:10.1111/cge.12395
McGivern MR, Best KE, Rankin J et al (2015) Epidemiology of congenital diaphragmatic hernia in Europe: a register-based study. Arch Dis Child Fetal Neonatal Ed 100:F137–F144. doi:10.1136/archdischild-2014-306174
Michaelson JJ, Shi Y, Gujral M et al (2012) Whole-genome sequencing in autism identifies hot spots for de novo germline mutation. Cell 151:1431–1442. doi:10.1016/j.cell.2012.11.019
Morrione A (2003) Grb10 adapter protein as regulator of insulin-like growth factor receptor signaling. J Cell Physiol 197(3):307–311
Nagata K, Masumoto K, Uesugi T et al (2007) Effect of insulin-like-growth factor and its receptors regarding lung development in fetal mice. Pediatr Surg Int 23(10):953–959
Neale BM, Kou Y, Liu L et al (2012) Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature 485:242–245. doi:10.1038/nature11011
Peters JM, Tedeschi A, Schmitz J (2008) The cohesin complex and its roles in chromosome biology. Genes Dev 22(22):3089–3114. doi:10.1101/gad.1724308
Pober BR (2007) Overview of epidemiology, genetics, birth defects, and chromosome abnormalities associated with CDH. Am J Med Genet C Semin Med Genet 145C:158–171. doi:10.1002/ajmg.c.30126
Pober BR (2008) Genetic aspects of human congenital diaphragmatic hernia. Clin Genet 74:1–15. doi:10.1111/j.1399-0004.2008.01031.x
Pober BR, Russell MK, Ackerman KG (2010) Congenital diaphragmatic hernia overview [GeneReviewsTM. 1993]. In: Pagon RA, Adam MP, Bird TD, Dolan CR, Fong CT, Smith RJH, Stephens K (eds) SourceGeneReviewsTM. Seattle Univ. Washington, Seattle, 1993–2013
Purcell S, Neale B, Todd-Brown K et al (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575. doi:10.1086/519795
Rivière J-B, van Bon BWM, Hoischen A et al (2012) De novo mutations in the actin genes ACTB and ACTG1 cause Baraitser-Winter syndrome. Nat Genet 44(440–4):S1–S2. doi:10.1038/ng.1091
Russell MK, Longoni M, Wells J et al (2012) Congenital diaphragmatic hernia candidate genes derived from embryonic transcriptomes. Proc Natl Acad Sci USA 109:2978–2983. doi:10.1073/pnas.1121621109
Samocha KE, Robinson EB, Sanders SJ et al (2014) A framework for the interpretation of de novo mutation in human disease. Nat Genet 46:944–950. doi:10.1038/ng.3050
Sanders SJ, Murtha MT, Gupta AR et al (2012) De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature 485:237–241. doi:10.1038/nature10945
Schrier SA, Sherer I, Deardorff MA et al (2011) Causes of death and autopsy findings in a large study cohort of individuals with Cornelia de Lange syndrome and review of the literature. Am J Med Genet A 155A(12):3007–3024. doi:10.1002/ajmg.a.34329
Sifrim A, Hitz M-P, Wilsdon A et al (2016) Distinct genetic architectures for syndromic and nonsyndromic congenital heart defects identified by exome sequencing. Nat Genet. doi:10.1038/ng.3627
Stolar CJH, Dillon PW (2012) Congenital diaphragmatic hernia and eventration. In: Pediatric surgery, 7th edn, pp 809–824
Sugiura N, Adams SM, Corriveau RA (2003) An evolutionarily conserved N-terminal acetyltransferase complex associated with neuronal development. J Biol Chem 278:40113–40120. doi:10.1074/jbc.M301218200
Veenma DCM, de Klein A, Tibboel D (2012) Developmental and genetic aspects of congenital diaphragmatic hernia. Pediatr Pulmonol 47:534–545. doi:10.1002/ppul.22553
Veltman JA, Brunner HG (2012) De novo mutations in human genetic disease. Nat Rev Genet 13:565–575. doi:10.1038/nrg3241
Verloes A, Di Donato N, Masliah-Planchon J et al (2015) Baraitser-Winter cerebrofrontofacial syndrome: delineation of the spectrum in 42 cases. Eur J Hum Genet 23:292–301. doi:10.1038/ejhg.2014.95
Ware JS, Samocha KE, Homsy J, Daly MJ (2015) Interpreting de novo Variation in Human Disease Using denovolyzeR. Curr Protoc Hum Genet 87:7.25.1–7.25.15. doi:10.1002/0471142905.hg0725s87
White J, Beck CR, Harel T et al (2016) POGZ truncating alleles cause syndromic intellectual disability. Genome Med 8:3. doi:10.1186/s13073-015-0253-0
Wolff G (1980) Familial congenital diaphragmatic defect: review and conclusions. Hum Genet 54:1–5
Wynn J, Yu L, Chung WK (2014) Genetic causes of congenital diaphragmatic hernia. Semin Fetal Neonatal Med 19:324–330. doi:10.1016/j.siny.2014.09.003
Xu B, Roos JL, Dexheimer P et al (2011) Exome sequencing supports a de novo mutational paradigm for schizophrenia. Nat Genet 43:864–868. doi:10.1038/ng.902
Ye J, Coulouris G, Zaretskaya I et al (2012) Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinform 13:134. doi:10.1186/1471-2105-13-134
Yu L, Wynn J, Cheung YH et al (2013) Variants in GATA4 are a rare cause of familial and sporadic congenital diaphragmatic hernia. Hum Genet 132:285–292. doi:10.1007/s00439-012-1249-0
Yu L, Bennett JT, Wynn J et al (2014) Whole exome sequencing identifies de novo mutations in GATA6 associated with congenital diaphragmatic hernia. J Med Genet. doi:10.1136/jmedgenet-2013-101989
Yu L, Sawle AD, Wynn J et al (2015) Increased burden of de novo predicted deleterious variants in complex congenital diaphragmatic hernia. Hum Mol Genet 24:4764–4773. doi:10.1093/hmg/ddv196
Zaidi S, Choi M, Wakimoto H et al (2013) De novo mutations in histone-modifying genes in congenital heart disease. Nature 498:220–223. doi:10.1038/nature12141
Zhu M, Yang T, Wei S et al (2003) Mutations in the gamma-actin gene (ACTG1) are associated with dominant progressive deafness (DFNA20/26). Am J Hum Genet 73:1082–1091. doi:10.1086/379286
Zhu X, Petrovski S, Xie P et al (2015) Whole-exome sequencing in undiagnosed genetic diseases: interpreting 119 trios. Genet Med 17:774–781. doi:10.1038/gim.2014.191
Acknowledgements
We thank Barbara R. Pober for her inspiration and her seminal contributions to the project, and the physicians at MassGeneral Hospital for Children and Boston Children’s Hospital for their continued support: T. Buchmiller, C. C. Chen, D. Doody, S. J. Fishman, A. Goldstein, L. Holmes, T. Jaksic, R. Jennings, C. Kelleher, D. Lawlor, C.W. Lillehei, P. Masiakos, D. P. Mooney, K. Papadakis, R. Pieretti, M. Puder, D. P. Ryan, R. C. Shamberger, C. Smithers, J. Vacanti, and C. Weldon. We are also grateful to all of the families at the participating Simons Simplex Collection (SSC) sites, as well as the principal investigators (A. Beaudet, R. Bernier, J. Constantino, E. Cook, E. Fombonne, D. Geschwind, R. Goin-Kochel, E. Hanson, D. Grice, A. Klin, D. Ledbetter, C. Lord, C. Martin, D. Martin, R. Maxim, J. Miles, O. Ousley, K. Pelphrey, B. Peterson, J. Piggot, C. Saulnier, M. State, W. Stone, J. Sutcliffe, C. Walsh, Z. Warren, E. Wijsman).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Financial Disclosure
Sequencing services were provided through the RS&G Service by the Northwest Genomics Center at the University of Washington, Department of Genome Sciences, under U.S. Federal Government contract number HHSN268201100037C from the National Heart, Lung, and Blood Institute (r223 to M. Longoni). Funding was provided by the National Institute of Child Health and Human Development (NICHD/NIH, http://www.nichd.nih.gov) P01HD068250. Partial funding was provided by the NICHD/NIH grant HD057036, by the Columbia University’s Clinical and Translational Science Award (CTSA), and by the National Center for Advancing Translational Sciences/National Institutes of Health (NCATS-NCRR/NIH, ncats.nih.gov) grant UL1 RR024156. Philanthropic funding was obtained by CHERUBS, the National Greek Orthodox Ladies Philoptochos Society, Inc., and generous donations from The Wheeler foundation, Vanech Family Foundation, Larsen Family, Wilke Family, and many other families. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
439_2017_1774_MOESM1_ESM.xlsx
Supplementary material 1 (XLSX 17 kb) Table S1 Clinical and genetic data for MGH/CHB Cohort. Patients are ordered according to Study ID. If previously published in the Longoni et al. 2014 publication, a conversion matrix to the alternative study ID is provided. Samples necessitating whole genome amplification prior to library construction are indicated. Patient data include self-reported ethnicity, gender, isolated or complex (syndromic) phenotype, side of the diaphragmatic defect, type of the diaphragmatic defect (Bochdalek, Morgagni, eventration, agenesis, or not otherwise specified)
439_2017_1774_MOESM2_ESM.docx
Supplementary material 2 (DOCX 14 kb) Table S2 WES metrics for MGH/CHB Cohort. Read length, reads per sample, median and mean coverage at each targeted base, and percent of targeted bases with at least 15X reads are indicated for each of the three sequencing batches (Yale, UW1, UW2) along with number of samples per each batch. Mean of samples and standard deviation are reported
439_2017_1774_MOESM3_ESM.xlsx
Supplementary material 3 (XLSX 74 kb) Table S3 De novo variants in combined MGH/CHB and DHREAMS cohorts. The complete and annotated list of variants is provided. Proband ID, study cohort and symbols of genes with de novo variants are indicated, as are position of the variants and official nomenclature according to Human Genome Variation Society (HGVS) recommendations
439_2017_1774_MOESM4_ESM.xlsx
Supplementary material 4 (XLSX 73 kb) Table S4 Enrichr enrichment terms. The list of genes with de novo variants was associated to functional biological terms in a systematic way for data exploration and interpretation using the integrative web-based application Enrichr. Significant enrichments in three libraries are shown (ChEA for transcription factor regulation, 2016 update; Biocarta for metabolic and signaling pathways, 2016 update; Reactome for curated biological pathways, 2016 update)
439_2017_1774_MOESM5_ESM.xlsx
Supplementary material 5 (XLSX 18 kb) Table S5 Annotated nodes in protein–protein interaction analysis (GeNets). Nodes are indicated in alphabetical order. Evidence for their implication in CDH is provided: de novo (genes with de novo variants in human cohorts), human (genetic variants in human cohorts except de novo), mouse (mouse models with diaphragmatic defects), bioinformatics (identified by expanding protein–protein networks to first and second order interactors). Community numbers indicate which interaction subnetwork, if any, the proteins belong to
439_2017_1774_MOESM6_ESM.xlsx
Supplementary material 6 (XLSX 14 kb) Table S6 Annotated modules (WGCNA) with significant enrichment for CDH genes. Modules are listed by arbitrary names (pink, yellow, brown, and blue). Total number of genes, number of CDH genes, and number of genes with de novo variants are shown for each module. Hypergeometric enrichment p values indicate whether each module contains more CDH genes than expected by random distribution
439_2017_1774_MOESM8_ESM.tif
Supplementary material 8 (TIFF 72 kb) Fig. S1 Poisson fit of de novo variant distribution in Boston (MGH/CHB) and DHREAMS cohorts. Number of patients with 0 ~ 7 de novo variants in the MGH/CHB (purple) and DHREAMS (cyan) cohorts are indicated. The superimposed lines indicate the expected distribution
439_2017_1774_MOESM9_ESM.tif
Supplementary material 9 (TIFF 254 kb) Fig. S2 Correlation between diaphragm and heart expression ranks for genes with de novo variants. The distribution of de novo variants is shown according to gene expression ranks in the developing diaphragm (x-axis) and in the developing heart (y-axis). Lines indicate the top quartile of expression rank. Genes are color coded according to functional consequence of the de novo variants (top left), cohort and patient phenotype (top right), type of LGD variant (bottom left), and functional prediction of missense variants compared to silent variants (bottom right)
439_2017_1774_MOESM10_ESM.tif
Supplementary material 10 (TIFF 147 kb) Fig. S3 Networks in protein–protein interaction analysis (GeNets). Additional network analysis of proteins with de novo variants: entire set (A), top quartile of expression rank in the developing diaphragm (B) and heart (C). GeNets (http://apps.broadinstitute.org/genets) was used for biological network analysis and visualization, implementing machine learning to identify previously unknown pathway relationships and discover functional modules based on the GeNets Metanetwork v1.0 combining protein–protein interaction information from InWeb3 and Gene Expression Networks from GEO
Rights and permissions
About this article
Cite this article
Longoni, M., High, F.A., Qi, H. et al. Genome-wide enrichment of damaging de novo variants in patients with isolated and complex congenital diaphragmatic hernia. Hum Genet 136, 679–691 (2017). https://doi.org/10.1007/s00439-017-1774-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-017-1774-y