Abstract
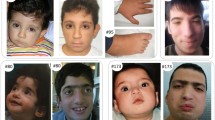
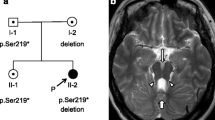
Rubinstein–Taybi syndrome (RSTS) is a distinct dominant disorder characterized by short stature, typical face, broad angulated thumbs and halluces, and mental retardation. The RSTS can be caused by chromosomal microdeletions and molecular mutations in the CREBBP gene; however, relatively few mutations have been reported to date. Here, we aimed to determine the rate of point mutations and other small molecular lesions in true RSTS and possible mild variants, by using genomic DNA sequencing. A consecutive series of patients including 17 patients from our previous study was investigated. We identified 19 causative mutations of CREBBP in a total of 45 patients representing three different diagnostic groups: (a) 17 mutations in 30 patients with unequivocal RSTS (detection rate 56.6%), (b) two mutations in eight patients with features suggestive of RSTS (“moderate or incomplete RSTS”, detection rate 25%), and (c) no mutation in seven patients with undiagnosed syndromes and isolated features of RSTS. In general, the mutations were distributed without hot spots and most were unique; however, three recurrent mutations (R370X, R1664H, and N1978S) were identified. Furthermore, we detected 15 different intragenic polymorphisms, including two non-synonymous coding polymorphisms, L551I and Q2208H. We report not only the highest detection rate (56.6%) of CREBBP mutations in patients with RSTS to date, but also the second missense mutation (N1978S) in a patient with moderate or incomplete RSTS. Previous studies have identified cytogenetic deletions in the CREBBP gene in eight to 12% of patients and very recently, Roelfsema et al. reported EP300 gene mutations in three of 92 (3.3%) patients with either true RSTS or different syndromes resembling RSTS. Our 56.6% detection rate of molecular mutations in CREBBP in patients with unequivocal RSTS supports the new concept that RSTS is a genetically heterogeneous disorder and furthermore, indicates that RSTS may be caused by gene/s other than CREBBP in up to 30% of cases.


Similar content being viewed by others
References
Bartsch O, Wagner A, Hinkel GK, Krebs P, Stumm M, Schmalenberger B, Böhm S, Balci S, Majewski F (1999) FISH studies in 45 patients with Rubinstein–Taybi syndrome: deletions associated with polysplenia, hypoplastic left heart and death in infancy. Eur J Hum Genet 7:748–756
Bartsch O, Locher K, Meinecke P, Kress W, Seemanová E, Wagner A, Ostermann K, Rödel G (2002) Molecular studies in 10 cases of Rubinstein–Taybi syndrome, including a mild variant showing a missense mutation in codon 1175 of CREBBP. J Med Genet 39:496–501
Cotsirilos P, Taylor JC, Matalon R (1987) Dominant inheritance of a syndrome similar to Rubinstein–Taybi. Am J Med Genet 26:85–93
Coupry I, Roudaut C, Stef M, Delrue MA, Marche M, Burgelin I, Taine L, Cruaud C, Lacombe D, Arveiler B (2002) Molecular analysis of the CBP gene in 60 patients with Rubinstein–Taybi syndrome. J Med Genet 39:416–421
Coupry I, Monnet L, Attia AA, Taine L, Lacombe D, Arveiler B (2004) Analysis of CBP (CREBBP) gene deletions in Rubinstein–Taybi syndrome patients using real-time quantitative PCR. Hum Mutat 23:278–284
Giles RH, Petrij F, Dauwerse HG, den Hollander AI, Lushnikova T, van Ommen GJ, Goodman RH, Deaven LL, Doggett NA, Peters DJ, Breuning MH (1997) Construction of a 1.2-Mb contig surrounding, and molecular analysis of, the human CREB-binding protein (CBP/CREBBP) gene on chromosome 16p13.3. Genomics 42:96–114
Goodman RH, Smolik S (2000) CBP/p300 in cell growth, transformation, and development. Genes Dev 14:1553–1577
Hendrich B, Bickmore W (2001) Human diseases with underlying defects in chromatin structure and modification. Hum Mol Genet 10:2233–2242
Hennekam RC, Van Den Boogaard MJ, Dijkstra PF, Van de Kamp JJ (1990a) Metacarpophalangeal pattern profile analysis in Rubinstein–Taybi syndrome. Am J Med Genet Suppl 6:48–50
Hennekam RC, Van Den Boogaard MJ, Sibbles BJ, Van Spijker HG (1990b) Rubinstein–Taybi syndrome in The Netherlands. Am J Med Genet Suppl 6:17–29
Kalkhoven E, Roelfsema JH, Teunissen H, den Boer A, Ariyurek Y, Zantema A, Breuning MH, Hennekam RC, Peters DJ (2003) Loss of CBP acetyltransferase activity by PHD finger mutations in Rubinstein–Taybi syndrome. Hum Mol Genet 12:441–450
King K, Flinter FA, Nihalani V, Green PM (2002) Unusual deep intronic mutations in the COL4A5 gene cause X linked Alport syndrome. Hum Genet 111:548–554
Kleinjan DA, van Heyningen V (2005) Long-range control of gene expression: emerging mechanisms and disruption in disease. Am J Hum Genet 76:8–32
Miller RW, Rubinstein JH (1995) Tumors in Rubinstein–Taybi syndrome. Am J Med Genet 56:112–115
Murata T, Kurokawa R, Krones A, Tatsumi K, Ishii M, Taki T, Masuno M, Ohashi H, Yanagisawa M, Rosenfeld MG, Glass CK, Hayashi Y (2001) Defect of histone acetyltransferase activity of the nuclear transcriptional coactivator CBP in Rubinstein–Taybi syndrome. Hum Mol Genet 10:1071–1076
Pagani F, Buratti E, Stuani C, Bendix R, Dörk T, Baralle FE (2002) A new type of mutation causes a splicing defect in ATM. Nat Genet 30:426–429
Petrij F, Giles RH, Dauwerse HG, Saris JJ, Hennekam RCM, Masuno M, Tommerup N, van Ommen G-JB, Goodman RH, Peters DJM, Breuning MH (1995) Rubinstein–Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature 376:348–351
Petrij F, Dauwerse HG, Blough RI, Giles RH, van der Smagt JJ, Wallerstein R, Maaswinkel-Mooy PD, van Karnebeek CD, van Ommen GJ, van Haeringen A, Rubinstein JH, Saal HM, Hennekam RC, Peters DJ, Breuning MH (2000) Diagnostic analysis of the Rubinstein–Taybi syndrome: five cosmids should be used for microdeletion detection and low number of protein truncating mutations. J Med Genet 37:168–176
Pöyhönen MH, Peippo MM, Valanne LK, Kuokkanen KE, Koskela SM, Bartsch O, Rasi S, Wiebe GJ, Kähkönen M, Kääriäinen HA (2004) Hypertrichosis, hyperkeratosis, abnormal corpus callosum, mental retardation and dysmorphic features in three unrelated females. Clin Dysmorphol 13:85–90
Roelfsema JH, White SJ, Ariyürek Y, Bartholdi D, Niedrist D, Papadia F, Bacino CA, den Dunnen JT, van Ommen GJB, Breuning MH, Hennekam RC, Peters DJM (2005) Genetic heterogeneity in Rubinstein–Taybi syndrome: mutations in both the CBP and EP300 genes can cause disease. Am J Hum Genet 76:572–580
Siraganian PA, Rubinstein JH, Miller RW (1989) Keloids and neoplasms in the Rubinstein–Taybi syndrome. Med Pediatr Oncol 17:485–491
Suminaga R, Takeshima Y, Adachi K, Yagi M, Nakamura H, Matsuo M (2002) A novel cryptic exon in intron 3 of the dystrophin gene was incorporated into dystrophin mRNA with a single nucleotide deletion in exon 5. J Hum Genet 47:196–201
Wiley S, Swayne S, Rubenstein JH, Lanphear NE, Stevens CA (2003) Rubinstein-Taybi syndrome medical guidelines. Am J Med Genet A 119:101–110
Acknowledgements
We would like to thank the patients and their families for contributing to this study, and our clinical colleagues for referring patients and clinical data, especially Prof Gabriele Gillessen-Kaesbach (University of Essen, Germany; patient 25), Professor Josef Kofer (Masaryk Hospital, Usti nad Labem, Czech Republic, patient 30), Professor Hans-Dieter-Rott (University of Erlangen, Germany; patient 33), Professor Marius Bembea and Dr. Cristina Skrypnyk (University of Oradea, Romania; patient 34), and Dr. Helen Hughes (North Wales Clinical Genetics Service, Rhyl, UK; patient 35). We would also like to thank Professor Peter Meinecke (Altonaer Kinderkrankenhaus, Hamburg, Germany) for interpreting the MCPP of patient 28, and Dr. Andreas Rump and Ms. Alice Schindler for comments. This work was supported in part by a grant (Ba 1397/5-1) from the Deutsche Forschungsgemeinschaft.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bartsch, O., Schmidt, S., Richter, M. et al. DNA sequencing of CREBBP demonstrates mutations in 56% of patients with Rubinstein–Taybi syndrome (RSTS) and in another patient with incomplete RSTS. Hum Genet 117, 485–493 (2005). https://doi.org/10.1007/s00439-005-1331-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00439-005-1331-y