Abstract
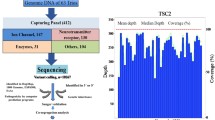
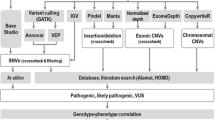
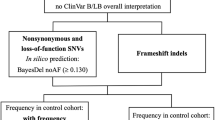
It is thought that despite highly variable phenotypic expression, 70—80% of all epileptic cases are caused by one or more genetic mutations. Next generation sequencing technologies, such as whole exome sequencing (WES), can be used in a diagnostic or research setting to identify genetic mutations which may have significant prognostic implications for patients and their families. In this study, 398 genes associated with epilepsy or recurrent seizures were stratified into tiers based on genotype–phenotype concordance, tissue gene expression, frequency of affected individuals with mutations and evidence from functional and family studies. WES was completed on 14 DNA samples (2 with known mutations in SCN1A and 12 with no known mutations) from individuals diagnosed with epilepsy using an Ion AmpliSeq approach. WES confirmed positive SCN1A mutations in two samples. In n = 5/12 samples (S-3 to -14) we identified potentially causative mutations across five different genes. S-5 was identified to have a novel missense mutation in CCM2; S-6 a novel frameshift mutation identified in ADGRV1; S-10 had a previously reported pathogenic mutation in PCDH19, whilst a novel missense mutation in PCDH19 was shown in S-12; and S-13 identified to have separate missense mutations in KCNA2 and NPRL3. The application of WES followed by a targeted variant prioritization approach allowed for the discovery of potentially causative mutations in our cohort of previously undiagnosed epilepsy patients.

Similar content being viewed by others
References
Akiyama M, Kobayashi K, Ohtsuka Y (2012) Dravet syndrome: a genetic epileptic disorder. Acta Med Okayama 66:369–376
Alexander A, Maroso M, Soltesz I (2016) Organization and control of epileptic circuits in temporal lobe epilepsy. Prog Brain Res 226:127–154
Annegers JF, Hauser WA, Anderson VE, Kurland LT (1982) The risks of seizure disorders among relatives of patients with childhood onset epilepsy. Neurology 32:174–179
Baldassari S, Licchetta L, Tinuper P, Bisulli F, Pippucci T (2016) GATOR1 complex: the common genetic actor in focal epilepsies. J Med Genet 53:503–510
Benton MC, Smith RA, Haupt LM, Sutherland HG, Dunn PJ, Albury CL et al (2019) Variant call format-diagnostic annotation and reporting tool: a customizable analysis pipeline for identification of clinically relevant genetic variants in next-generation sequencing data. J Mol Diagn 21(6):951–960
Bromfield EB, Cavazos JE, Sirven JI (eds) (2006) An introduction to epilepsy [Internet]. In: Basic mechanisms underlying seizures and epilepsy, chap 1. American Epilepsy Society, West Hartford (CT). https://www.ncbi.nlm.nih.gov/books/NBK2510/
Cunningham K, Uchida Y, O'Donnell E, Claudio E, Li W, Soneji K, Wang H, Mukouyama YS, Siebenlist U (2011) Conditional deletion of Ccm2 causes hemorrhage in the adult brain: a mouse model of human cerebral cavernous malformations. Hum Mol Genet 20:3198–3206
D'Angelo R, Marini V, Rinaldi C, Origone P, Dorcaratto A, Avolio M, Goitre L, Forni M, Capra V, Alafaci C, Mareni C, Garre C, Bramanti P, Sidoti A, Retta SF, Amato A (2011) Mutation analysis of CCM1, CCM2 and CCM3 genes in a cohort of Italian patients with cerebral cavernous malformation. Brain Pathol 21:215–224
Delahaye-Duriez A, Srivastava P, Shkura K, Langley SR, Laaniste L, Moreno-Moral A, Danis B, Mazzuferi M, Foerch P, Gazina EV, Richards K, Petrou S, Kaminski RM, Petretto E, Johnson MR (2016) Rare and common epilepsies converge on a shared gene regulatory network providing opportunities for novel antiepileptic drug discovery. Genome Biol 17:245
Depienne C, Bouteiller D, Keren B, Cheuret E, Poirier K, Trouillard O, Benyahia B, Quelin C, Carpentier W, Julia S, Afenjar A, Gautier A, Rivier F, Meyer S, Berquin P, Helias M, Py I, Rivera S, Bahi-Buisson N, Gourfinkel-An I, Cazeneuve C, Ruberg M, Brice A, Nabbout R, Leguern E (2009) Sporadic infantile epileptic encephalopathy caused by mutations in PCDH19 resembles Dravet syndrome but mainly affects females. PLoS Genet 5:e1000381
Depienne C, LeGuern E (2012) PCDH19-related infantile epileptic encephalopathy: an unusual X-linked inheritance disorder. Hum Mutat 33:627–634
Dibbens LM, Heron SE, Mulley JC (2007) A polygenic heterogeneity model for common epilepsies with complex genetics. Genes Brain Behav 6:593–597
Dibbens LM, Tarpey PS, Hynes K, Bayly MA, Scheffer IE, Smith R, Bomar J, Sutton E, Vandeleur L, Shoubridge C, Edkins S, Turner SJ, Stevens C, O'Meara S, Tofts C, Barthorpe S, Buck G, Cole J, Halliday K, Jones D, Lee R, Madison M, Mironenko T, Varian J, West S, Widaa S, Wray P, Teague J, Dicks E, Butler A, Menzies A, Jenkinson A, Shepherd R, Gusella JF, Afawi Z, Mazarib A, Neufeld MY, Kivity S, Lev D, Lerman-Sagie T, Korczyn AD, Derry CP, Sutherland GR, Friend K, Shaw M, Corbett M, Kim HG, Geschwind DH, Thomas P, Haan E, Ryan S, McKee S, Berkovic SF, Futreal PA, Stratton MR, Mulley JC, Gecz J (2008) X-linked protocadherin 19 mutations cause female-limited epilepsy and cognitive impairment. Nat Genet 40:776–781
Dunn P, Albury CL, Maksemous N, Benton MC, Sutherland HG, Smith RA, Haupt LM, Griffiths LR (2018) Next generation sequencing methods for diagnosis of epilepsy syndromes. Front Genet 9:20
Epi KC (2012) Epi4K: gene discovery in 4000 genomes. Epilepsia 53:1457–1467
Escayg A, Goldin AL (2010) Sodium channel SCN1A and epilepsy: mutations and mechanisms. Epilepsia 51:1650–1658
Fisher KJ, Aronson NN Jr (1991) Characterization of the mutation responsible for aspartylglucosaminuria in three finnish patients. amino acid substitution Cys163––Ser abolishes the activity of lysosomal glycosylasparaginase and its conversion into subunits. J Biol Chem 266:12105–12113
Fujiwara T, Sugawara T, Mazaki-Miyazaki E, Takahashi Y, Fukushima K, Watanabe M, Hara K, Morikawa T, Yagi K, Yamakawa K, Inoue Y (2003) Mutations of sodium channel α subunit type 1 (SCN1A) in intractable childhood epilepsies with frequent generalized tonic–clonic seizures. Brain 126:531–546
Fukata Y, Fukata M (2017) Epilepsy and synaptic proteins. Curr Opin Neurobiol 45:1–8
Gagliardi M, Annesi G, Sesta M, Tarantino P, Conti P, Labate A, Di Rosa G, Quattrone A, Gambardella A (2015) PCDH19 mutations in female patients from Southern Italy. Seizure 24:118–120
Garofalo S, Cornacchione M, Di Costanzo A (2012) From genetics to genomics of epilepsy. Neurol Res Int 2012:18
Ghali MG, Srinivasan VM, Mohan AC, Jones JY, Kan PT, Lam S (2016) Pediatric cerebral cavernous malformations: genetics, pathogenesis, and management. Surg Neurol Int 7:S1127–S1134
Giraldez BG, Guerrero-Lopez R, Ortega-Moreno L, Verdu A, Carrascosa-Romero MC, Garcia-Campos O, Garcia-Munozguren S, Pardal-Fernandez JM, Serratosa JM (2015) Uniparental disomy as a cause of spinal muscular atrophy and progressive myoclonic epilepsy: phenotypic homogeneity due to the homozygous c.125C%3eT mutation in ASAH1. Neuromuscul Disord 25:222–224
Hattori J, Ouchida M, Ono J, Miyake S, Maniwa S, Mimaki N, Ohtsuka Y, Ohmori I (2008) A Screening test for the prediction of Dravet syndrome before one year of age. Epilepsia 49:626–633
Henshall DC, Kobow K (2015) Epigenetics and epilepsy. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a022731
Higurashi N, Nakamura M, Sugai M, Ohfu M, Sakauchi M, Sugawara Y, Nakamura K, Kato M, Usui D, Mogami Y, Fujiwara Y, Ito T, Ikeda H, Imai K, Takahashi Y, Nukui M, Inoue T, Okazaki S, Kirino T, Tomonoh Y, Inoue T, Takano K, Shimakawa S, Hirose S (2013) PCDH19-related female-limited epilepsy: further details regarding early clinical features and therapeutic efficacy. Epilepsy Res 106:191–199
Hirano S, Takeichi M (2012) Cadherins in brain morphogenesis and wiring. Physiol Rev 92:597–634
Hundallah K, Aa A, AlHashem A, Tabarki B (2016) Severe early-onset epileptic encephalopathy due to mutations in the KCNA2 gene: expansion of the genotypic and phenotypic spectrum. Euro J Paediatric Neurol 20:657–660
Kaartinen V, Mononen I, Voncken JW, Noronkoski T, Gonzalez-Gomez I, Heisterkamp N, Groffen J (1996) A mouse model for the human lysosomal disease aspartylglycosaminuria. Nat Med 2:1375–1378
Kearney JA (2015) KCNA2-related epileptic encephalopathy. Pediatr Neurol Briefs 29:27
Korenke G, Eggert M, Thiele H, Nürnberg P, Sander T, Steinlein OK (2016) Nocturnal frontal lobe epilepsy caused by a mutation in the GATOR1 complex gene NPRL3. Epilepsia 57:e60–e63. https://doi.org/10.1111/epi.13307
Lerche H, Shah M, Beck H, Noebels J, Johnston D, Vincent A (2013) Ion channels in genetic and acquired forms of epilepsy. J Physiol 591:753–764
Liu A, Xu X, Yang X, Jiang Y, Yang Z, Liu X, Wu Y, Wu X, Wei L, Zhang Y (2017) The clinical spectrum of female epilepsy patients with PCDH19 mutations in a Chinese population. Clin Genet 91:54–62
Lohmann K, Klein C (2014) Next generation sequencing and the future of genetic diagnosis. Neurotherapeutics 11:699–707
Madrigal I, Alvarez-Mora MI, Karlberg O, Rodriguez-Revenga L, Elurbe DM, Rabionet R, Mur A, Pie J, Ballesta F, Sauer S, Syvanen AC, Mila M (2014) Efficient application of next-generation sequencing for the diagnosis of rare genetic syndromes. J Clin Pathol 67:1099–1103
Martin HC, Kim GE, Pagnamenta AT, Murakami Y, Carvill GL, Meyer E, Copley RR, Rimmer A, Barcia G, Fleming MR, Kronengold J, Brown MR, Hudspith KA, Broxholme J, Kanapin A, Cazier JB, Kinoshita T, Nabbout R, Consortium WGS, Bentley D, McVean G, Heavin S, Zaiwalla Z, McShane T, Mefford HC, Shears D, Stewart H, Kurian MA, Scheffer IE, Blair E, Donnelly P, Kaczmarek LK, Taylor JC (2014) Clinical whole-genome sequencing in severe early-onset epilepsy reveals new genes and improves molecular diagnosis. Hum Mol Genet 23:3200–3211
Masnada S, Hedrich UBS, Gardella E, Schubert J, Kaiwar C, Klee EW, Lanpher BC, Gavrilova RH, Synofzik M, Bast T, Gorman K, King MD, Allen NM, Conroy J, Ben Zeev B, Tzadok M, Korff C, Dubois F, Ramsey K, Narayanan V, Serratosa JM, Giraldez BG, Helbig I, Marsh E, O’Brien M, Bergqvist CA, Binelli A, Porter B, Zaeyen E, Horovitz DD, Wolff M, Marjanovic D, Caglayan HS, Arslan M, Pena SDJ, Sisodiya SM, Balestrini S, Syrbe S, Veggiotti P, Lemke JR, Møller RS, Lerche H, Rubboli G (2017) Clinical spectrum and genotype–phenotype associations of KCNA2-related encephalopathies. Brain 140:2337–2354
McDonald DA, Shenkar R, Shi C, Stockton RA, Akers AL, Kucherlapati MH, Kucherlapati R, Brainer J, Ginsberg MH, Awad IA, Marchuk DA (2011) A novel mouse model of cerebral cavernous malformations based on the two-hit mutation hypothesis recapitulates the human disease. Hum Mol Genet 20:211–222
McMillan DR, Kayes-Wandover KM, Richardson JA, White PC (2002) Very large G protein-coupled receptor-1, the largest known cell surface protein, is highly expressed in the developing central nervous system. J Biol Chem 277:785–792
Mefford HC (2015) Copy number matters in epilepsy. Epilepsy Curr 15:180–182
Miller IO, Sotero de Menezes MA (1993–2020) SCN1A seizure disorders. In: Adam MP, Ardinger HH, Pagon RA et al (eds) GeneReviews® [Internet], University of Washington, Seattle, WA. https://www.ncbi.nlm.nih.gov/books/NBK1318/
Mullen SA, Carvill GL, Bellows S, Bayly MA, Trucks H, Lal D, Sander T, Berkovic SF, Dibbens LM, Scheffer IE, Mefford HC (2013) Copy number variants are frequent in genetic generalized epilepsy with intellectual disability. Neurology 81:1507–1514
Mulley JC, Scheffer IE, Petrou S, Berkovic SF (2003) Channelopathies as a genetic cause of epilepsy. Curr Opin Neurol 16:171–176
Myers CT, Mefford HC (2015) Advancing epilepsy genetics in the genomic era. Genome Med 7:91
Myers KA, Nasioulas S, Boys A, McMahon JM, Slater H, Lockhart P, Sart Dd, Scheffer IE (2018) ADGRV1 is implicated in myoclonic epilepsy. Epilepsia 59:381–388
Nakayama J, Fu YH, Clark AM, Nakahara S, Hamano K, Iwasaki N, Matsui A, Arinami T, Ptacek LJ (2002) A nonsense mutation of the MASS1 gene in a family with febrile and afebrile seizures. Ann Neurol 52:654–657
Ng BG, Sharma V, Sun L, Loh E, Hong W, Tay SK, Freeze HH (2011) Identification of the first COG-CDG patient of Indian origin. Mol Genet Metab 102:364–367
Noh GJ, Jane Tavyev Asher Y, Graham JM Jr (2012) Clinical review of genetic epileptic encephalopathies. Eur J Med Genet 55:281–298
Olson H, Shen Y, Avallone J, Sheidley BR, Pinsky R, Bergin AM, Berry GT, Duffy FH, Eksioglu Y, Harris DJ, Hisama FM, Ho E, Irons M, Jacobsen CM, James P, Kothare S, Khwaja O, Lipton J, Loddenkemper T, Markowitz J, Maski K, Megerian JT, Neilan E, Raffalli PC, Robbins M, Roberts A, Roe E, Rollins C, Sahin M, Sarco D, Schonwald A, Smith SE, Soul J, Stoler JM, Takeoka M, Tan WH, Torres AR, Tsai P, Urion DK, Weissman L, Wolff R, Wu BL, Miller DT, Poduri A (2014) Copy number variation plays an important role in clinical epilepsy. Ann Neurol 75:943–958
Opladen T, Ebinger F, Zschocke J, Sengupta D, Ben-Omran T, Shahbeck N, Moog U, Fischer C, Burger F, Haas D, Ruef P, Harting I, Al-Rifai H, Hoffmann GF (2014) Aspartylglucosaminuria: unusual neonatal presentation in Qatari twins with a novel aspartylglucosaminidase gene mutation and 3 new cases in a Turkish family. J Child Neurol 29:36–42
Pederick DT, Homan CC, Jaehne EJ, Piltz SG, Haines BP, Baune BT, Jolly LA, Hughes JN, Gecz J, Thomas PQ (2016) Pcdh19 loss-of-function increases neuronal migration in vitro but is dispensable for brain development in mice. Sci Rep 6:26765
Poduri A, Lowenstein D (2011) Epilepsy genetics–past, present, and future. Curr Opin Genet Dev 21:325–332
Poduri A, Sheidley BR, Shostak S, Ottman R (2014) Genetic testing in the epilepsies-developments and dilemmas. Nat Rev Neurol 10:293–299
Ragona F, Brazzo D, De Giorgi I, Morbi M, Freri E, Teutonico F, Gennaro E, Zara F, Binelli S, Veggiotti P, Granata T (2010) Dravet syndrome: early clinical manifestations and cognitive outcome in 37 Italian patients. Brain Dev 32:71–77
Rahman S (2012) Mitochondrial disease and epilepsy. Dev Med Child Neurol 54:397–406
Ran X, Li J, Shao Q, Chen H, Lin Z, Sun ZS, Wu J (2015) EpilepsyGene: a genetic resource for genes and mutations related to epilepsy. Nucleic Acids Res 43:D893–899
Reynders E, Foulquier F, Leao Teles E, Quelhas D, Morelle W, Rabouille C, Annaert W, Matthijs G (2009) Golgi function and dysfunction in the first COG4-deficient CDG type II patient. Hum Mol Genet 18:3244–3256
Rhodes TH, Vanoye CG, Ohmori I, Ogiwara I, Yamakawa K, George AL Jr (2005) Sodium channel dysfunction in intractable childhood epilepsy with generalized tonic-clonic seizures. J Physiol 569:433–445
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med 17:405–424
Roopra A, Dingledine R, Hsieh J (2012) Epigenetics and epilepsy. Epilepsia 53(Suppl 9):2–10
Rosenow F, Alonso-Vanegas MA, Baumgartner C, Blumcke I, Carreno M, Gizewski ER, Hamer HM, Knake S, Kahane P, Luders HO, Mathern GW, Menzler K, Miller J, Otsuki T, Ozkara C, Pitkanen A, Roper SN, Sakamoto AC, Sure U, Walker MC, Steinhoff BJ, CoTSotI STF (2013) Cavernoma-related epilepsy: review and recommendations for management–report of the surgical task force of the ILAE commission on therapeutic strategies. Epilepsia 54:2025–2035
Saarela J, Laine M, Oinonen C, von Schantz C, Jalanko A, Rouvinen J, Peltonen L (2001) Molecular pathogenesis of a disease: structural consequences of aspartylglucosaminuria mutations. Hum Mol Genet 10:983–995
Saito S, Ohno K, Sugawara K, Suzuki T, Togawa T, Sakuraba H (2008) Structural basis of aspartylglucosaminuria. Biochem Biophys Res Commun 377:1168–1172
Scheffer IE, Berkovic SF (1997) Generalized epilepsy with febrile seizures plus. A genetic disorder with heterogeneous clinical phenotypes. Brain 120(Pt 3):479–490
Scheffer IE, Berkovic SF (2003) The genetics of human epilepsy. Trends Pharmacol Sci 24:428–433
Scheffer IE, Turner SJ, Dibbens LM, Bayly MA, Friend K, Hodgson B, Burrows L, Shaw M, Wei C, Ullmann R, Ropers HH, Szepetowski P, Haan E, Mazarib A, Afawi Z, Neufeld MY, Andrews PI, Wallace G, Kivity S, Lev D, Lerman-Sagie T, Derry CP, Korczyn AD, Gecz J, Mulley JC, Berkovic SF (2008) Epilepsy and mental retardation limited to females: an under-recognized disorder. Brain 131:918–927
Sparks SE, Krasnewich DM (1993–2020) Congenital disorders of N-linked glycosylation and multiple pathway overview. In: Adam MP, Ardinger HH, Pagon RA et al (eds) GeneReviews®, University of Washington, Seattle, WA. https://www.ncbi.nlm.nih.gov/books/NBK1332/
Spillane J, Kullmann DM, Hanna MG (2016) Genetic neurological channelopathies: molecular genetics and clinical phenotypes. J Neurol Neurosurg Psychiatry 87:37–48
Staub E, Perez-Tur J, Siebert R, Nobile C, Moschonas NK, Deloukas P, Hinzmann B (2002) The novel EPTP repeat defines a superfamily of proteins implicated in epileptic disorders. Trends Biochem Sci 27:441–444
Sui L, Lakshminarasimhan D, Pande S, Guo HC (2014) Structural basis of a point mutation that causes the genetic disease aspartylglucosaminuria. Structure 22:1855–1861
Sun Y, Ruivenkamp CA, Hoffer MJ, Vrijenhoek T, Kriek M, van Asperen CJ, den Dunnen JT, Santen GW (2015) Next-generation diagnostics: gene panel, exome, or whole genome? Hum Mutat 36:648–655
Syrbe S, Hedrich UBS, Riesch E, Djemie T, Muller S, Moller RS, Maher B, Hernandez-Hernandez L, Synofzik M, Caglayan HS, Arslan M, Serratosa JM, Nothnagel M, May P, Krause R, Loffler H, Detert K, Dorn T, Vogt H, Kramer G, Schols L, Mullis PE, Linnankivi T, Lehesjoki AE, Sterbova K, Craiu DC, Hoffman-Zacharska D, Korff CM, Weber YG, Steinlin M, Gallati S, Bertsche A, Bernhard MK, Merkenschlager A, Kiess W, Euro ERESc, Gonzalez M, Zuchner S, Palotie A, Suls A, De Jonghe P, Helbig I, Biskup S, Wolff M, Maljevic S, Schule R, Sisodiya SM, Weckhuysen S, Lerche H, Lemke JR (2015) De novo loss- or gain-of-function mutations in KCNA2 cause epileptic encephalopathy. Nat Genet 47: 393–399
Tan EH, Yusoff AAM, Abdullah JM, Razak SA (2012) Generalized epilepsy with febrile seizure plus (GEFS+) spectrum: novel de novo mutation of SCN1A detected in a Malaysian patient. J Pediatric Neurosci 7:123–125
Trivisano M, Pietrafusa N, Ciommo V, Cappelletti S, Palma L, Terracciano A, Bertini E, Vigevano F, Specchio N (2016) PCDH19-related epilepsy and Dravet syndrome: face-off between two early-onset epilepsies with fever sensitivity. Epilepsy Res 125:32–36
Wang JW, Shi XY, Kurahashi H, Hwang SK, Ishii A, Higurashi N, Kaneko S, Hirose S, Epilepsy Genetic Study Group J (2012) Prevalence of SCN1A mutations in children with suspected Dravet syndrome and intractable childhood epilepsy. Epilepsy Res 102:195–200
Wang Y, Fan X, Zhang W, Zhang C, Wang J, Jiang T, Wang L (2015) Deficiency of very large G-protein-coupled receptor-1 is a risk factor of tumor-related epilepsy: a whole transcriptome sequencing analysis. J Neurooncol 121:609–616
Zhang W, Cui H, Wong LJ (2014) Application of next generation sequencing to molecular diagnosis of inherited diseases. Top Curr Chem 336:19–45
Funding
This work was supported by Corbett philanthropic research funding and utilized infrastructure purchased with Australian Government EIF Super Science Funds as part of the Therapeutic Innovation Australia—Queensland Node Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
PJD, BHM, CLA, SS, HGS, NM, MCB, RAS, LMH and LRG declare that they have no conflict of interest.
Ethical approval
All procedures in the study involving informed consent for human participants were performed in accordance with ethical standards approved by Queensland University of Technology human research ethics committee (Approval No. 1400000748) and within the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Communicated by Stefan Hohmann.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Dr. Cassie Albury has recently moved employment to Abacus Dx, Dr. Miles Benton has moved employment to the Institute of Environmental Science and Research Ltd (ESR) and Dr. Shani Stuart is now employed at the Peter McCallum Cancer Centre.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Dunn, P.J., Maher, B.H., Albury, C.L. et al. Tiered analysis of whole-exome sequencing for epilepsy diagnosis. Mol Genet Genomics 295, 751–763 (2020). https://doi.org/10.1007/s00438-020-01657-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-020-01657-x