Abstract
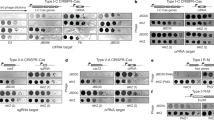
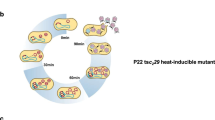
Bacteriophage λ genome is one of the classical model replicons in studies on the regulation of DNA replication. Moreover, since genes coding for Shiga toxins are located in genomes of lambdoid phages, understanding of mechanisms controlling λ DNA replication may be of bio-medical importance. During lytic development of bacteriophage λ, its genome is replicated according to the θ (circle-to-circle) mode early after infection, and then it is switched to the σ (rolling circle) mode. Two mechanisms of regulation of this switch were proposed recently and both suggested a crucial role for directionality of λ DNA replication. Whereas one hypothesis assumed transient impairment of ClpP/ClpX-mediated proteolysis of the λO initiator protein, another suggested a crucial role for transcriptional activation of the oriλ region and factors involved in the control of the p R promoter activity. Here we demonstrate that mutations in clpP and clpX genes had little influence on both directionality of λ DNA replication and appearance of σ replication intermediates. On the other hand, regulators affecting activity of the p R promoter (responsible for initiation of transcription, which activates oriλ) directly or indirectly influenced directionality of λ DNA replication to various extents. Therefore, we conclude that regulation of the efficiency of transcriptional activation of oriλ, rather than transient impairment of the λO proteolysis, is responsible for the control of the switch from θ to σ replication, and propose a model for this control.



Similar content being viewed by others
References
Alfano C, McMacken R (1989a) Ordered assembly of nucleoprotein structures at the bacteriophage lambda replication origin during the initiation of DNA replication. J Biol Chem 264:10699–10708
Alfano C, McMacken R (1989b) Heat shock protein-mediated disassembly of nucleoprotein structures is required for initiation of bacteriophage λ DNA replication. J Biol Chem 264:10709–10718
Barańska S, Gabig M, Węgrzyn A, Konopa G, Herman-Antosiewicz A, Hernandez P, Schvartzman JB, Helinski DR, Węgrzyn G (2001) Regulation of the switch from early to late bacteriophage λ DNA replication mode. Microbiology 147:535–547
Barańska S, Konopa G, Węgrzyn G (2002) Directionality of λ plasmid DNA replication carried out by the heritable replication complex. Nucleic Acids Res 30:1176–1181
Bejarano I, Klemes Y, Schoulaker-Schwarz R, Engelberg-Kulka H (1993) Energy-dependent degradation of λO protein in Escherichia coli. J Bacteriol 175:7720–7723
Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heyneker HL, Boyer HW (1977) Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113
Bull HJ (1995) Bacteriophage lambda replication—coupled processes, genetic elements and regulatory choices. PhD Thesis, University of Saskatchewan, Canada
Burkardt H, Lurz R (1984) Electron microscopy. In: Puhler A, Timmis KN (eds) Advanced molecular genetics. Srpinger, Berlin, pp 281–313
Czyż A, Zielke R, Węgrzyn G (2001) Rapid degradation of bacteriophage λ O protein by ClpP/ClpX protease influences the lysis-versus-lysogenization decision of the phage under certain growth conditions of the host cells. Arch Virol 146:1487–1498
Datta I, Banik-Maiti S, Adhikari L, Sau S, Das N, Mandal NC (2005a) The mutation that makes Escherichia coli resistant to λ P gene-mediated host lethality is located within DNA initiator gene dnaA of the bacterium. J Biochem Mol Biol 38:89–96
Datta I, Sau S, Sil AK, Mandal NC (2005b) The bacteriophage λ DNA replication protein P inhibits the oriC DNA- and ATP-binding functions of the DNA replication initiator protein DnaA of Escherichia coli. J Biochem Mol Biol 38:97–103
Dodson M, Echols H, Wickner S, Alfano C, Mensa-Wilmot K, Gomes B, LeBowitz J, Roberts JD, McMacken R (1986) Specialized nucleoprotein structures at the origin of replication of bacteriophage λ: localized unwinding of duplex DNA by a six protein reaction. Proc Natl Acad Sci USA 83:7638–7642
Friedman DI, Court DL (2001) Bacteriophage lambda: alive and well and still doing its thing. Curr Opin Microbiol 4:201–207
Glinkowska M, Majka J, Messer W, Węgrzyn G (2003) The mechanism of regulation of bacteriophage λ p R promoter activity by Escherichia coli DnaA protein. J Biol Chem 278:22250–22256
Gottesman S, Clark WP, de Crecy-Lagard V, Maurizi MR (1993) ClpX, an alternative subunit for the ATP-dependent Clp protease of Escherichia coli. J Biol Chem 268:22618–22626
Herman-Antosiewicz A, Śrutkowska S, Taylor K, Węgrzyn G (1998) Replication and maintenance of λ plasmids devoid of the Cro repressor autoregulatory loop in Escherichia coli. Plasmid 40:113–125
Jensen KF (1993) The Escherichia coli “wild types” W3110 and MG1655 have an rph frameshift mutation that leads to pyrimidine starvation due to low pyrE expression levels. J Bacteriol 175:3401–3407
Kiger JA Jr, Sinsheimer RL (1971) DNA of vegetative bacteriophage lambda, VI. Electron microscopic studies of replicating lambda DNA. Proc Natl Acad Sci USA 68:112–115
Konopa G, Barańska S, Węgrzyn A, Węgrzyn G (2000) Bacteriophage and host mutants causing the rolling-circle λ DNA replication early after infection. FEBS Lett 472:217–220
Kur J, Górska I, Taylor K (1987) Escherichia coli dnaA initiation function is required for replication of plasmids derived from coliphage lambda. J Mol Biol 198:203–210
Learn B, Karzai AW, McMacken R (1993) Transcription stimulates the establishment of bidirectional λ DNA replication in vitro. Cold Spring Harbor Symp Quant Biol 58:389–402
Mensa-Wilmot K, Seaby R, Alfano C, Wold MS, Gomes B, McMacken R (1989) Reconstitution of a nine-protein system that initiates bacteriophage λ DNA replication. J Biol Chem 264:2853–2861
Reuben RC, Skalka A (1977) Identification of the site of interruption in relaxed circles producing during bacteriophage λ DNA circle replication. J Virol 21:673–682
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory mannual. Cold Spring Harbor laboratory press, Cold Spring Harbor
Śrutkowska S, Konopa G, Węgrzyn G (1998) A method for isolation of plasmid DNA replication intermediates from unsynchronized bacterial cultures for electron microscopy analysis. Acta Biochim Pol 45:233–240
Śrutkowska S, Caspi R, Gabig M, Węgrzyn G (1999) Detection of DNA replication intermediates after two-dimensional agarose gel electrophoresis using a fluorescein-labeled probe. Anal Biochem 269:221–222
Szalewska-Pałasz A, Węgrzyn A, Błaszczak A, Taylor K, Węgrzyn G (1998a) DnaA-stimulated transcriptional activation of oriλ: Escherichia coli RNA polymerase β subunit as a transcriptional activator contact site. Proc Natl Acad Sci USA 95:4241–4246
Szalewska-Pałasz A, Weigel C, Speck C, Śrutkowska S, Konopa G, Lurz R, Marszałek J, Taylor K, Messer W, Węgrzyn G (1998b) Interaction of the Escherichia coli DnaA protein with bacteriophage λ DNA. Mol Gen Genet 259:679–688
Takahashi S (1975) The starting point and direction of rolling-circle replicative intermediates of coliphage λ DNA. Mol Gen Genet 142:137–153
Takahashi S (1977) Rolling circle replicative structure of bacteriophage λ DNA in a recombination deficient system. Mol Gen Genet 152:201–204
Taylor K, Węgrzyn G (1995) Replication of coliphage lambda DNA. FEMS Microbiol Rev 17:109–119
Taylor K, Węgrzyn G (1998) Regulation of bacteriophage λ replication. In: Busby SJW, Thomas CMN, Brown L (eds) Molecular microbiology. Springer, Berlin, pp 81–97
Viguera E, Hernandez P, Krimer DB, Boistov AS, Lurz R, Alonso JC, Schvartzman JB (1996) The ColE1 unidirectional origin acts as a polar replication fork pausing site. J Biol Chem 271:22414–22421
Viguera E, Rodríguez A, Krimer DB, Hernández P, Trelles O, Schvartzman JB (1998) A computer model for the analysis of DNA replication intermediates by two-dimensional (2D) agarose gel electrophoresis. Gene 217:41–49
Waldor MK, Friedman DI (2005) Phage regulatory circuits and virulence gene expression. Curr Opin Microbiol 8:459–465
Węgrzyn G, Taylor K (1992) Inheritance of the replication complex by one of two daughter copies during λ plasmid replication in Escherichia coli. J Mol Biol 226:681–688
Węgrzyn A, Węgrzyn G (2001) Inheritance of the replication complex: a unique or common phenomenon in the control of DNA replication? Arch Microbiol 175:86–93
Węgrzyn G, Węgrzyn A (2002) Stress responses and replication of plasmids in bacterial cells. Microb Cell Factor 1:2
Węgrzyn G, Węgrzyn A (2005) Genetic switches during bacteriophage lambda development. Prog Nucleic Acid Res Mol Biol 79:1–48
Węgrzyn G, Pawłowicz A, Taylor K (1992) Stability of coliphage λ DNA replication initiator, the λO protein. J Mol Biol 226:675–680
Węgrzyn A, Węgrzyn G, Taylor K (1995) Plasmid and host functions required for λ plasmid replication carried out by the inherited replication complex. Mol Gen Genet 247:501–508
Węgrzyn G, Szalewska-Pałasz A, Węgrzyn A, Obuchowski M, Taylor K (1995a) Transcriptional activation of the origin of coliphage λ DNA replication is regulated by the host DnaA initiator function. Gene 154:47–50
Węgrzyn G, Węgrzyn A, Konieczny I, Bielawski K, Konopa G, Obuchowski M, Helinski DR, Taylor K (1995b) Involvement of the host initiator function dnaA in the replication of coliphage λ. Genetics 139:1469–1481
Węgrzyn A, Węgrzyn G, Herman A, Taylor K (1996) Protein inheritance: λ plasmid replication perpetuated by the heritable replication complex. Genes Cells 1:953–963
Węgrzyn A, Czyż A, Gabig M, Węgrzyn G (2000) ClpP/ClpX-mediated degradation of the bacteriophage λ O protein and regulation of λ phage and λ plasmid replication. Arch Microbiol 174:89–96
Węgrzyn G, Węgrzyn A, Barańska S, Czyż A (2001) Regulation of bacteriophage lambda development. Recent Res Dev Virol 3:375–386
Weigel C, Seitz H (2006) Bacteriophage replication modules. FEMS Microbiol Rev 30:321–381
Wojtkowiak D, Georgopoulos C, Żylicz M (1993) Isolation and characterization of ClpX, a new ATP-dependent specificity component of the Clp protease of Escherichia coli. J Biol Chem 268:22609–22617
Żylicz M, Ang D, Liberek K, Georgopoulos C (1989) Initiation of λ DNA replication with purified host- and bacteriophage-encoded proteins: role of the DnaK, DnaJ and GrpE heat shock proteins. EMBO J 8:1601–1608
Żylicz M, Liberek K, Wawrzynów A, Georgopoulos C (1998) Formation of the preprimosome protects λ O from RNA transcription-dependent proteolysis by ClpP/ClpX. Proc Natl Acad Sci USA 95:15259–15263
Acknowledgments
Assistance of Elżbieta Borysiewicz during some experiments is greatly acknowledged. This work was supported by the Ministry of Science and Higher Education (project grant no. N301 122 31/3747).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G. Klug.
Magdalena Narajczyk and Sylwia Barańska contributed equally to this work.
Rights and permissions
About this article
Cite this article
Narajczyk, M., Barańska, S., Węgrzyn, A. et al. Switch from θ to σ replication of bacteriophage λ DNA: factors involved in the process and a model for its regulation. Mol Genet Genomics 278, 65–74 (2007). https://doi.org/10.1007/s00438-007-0228-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-007-0228-y