Abstract
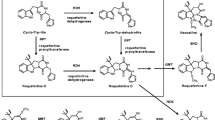
A putative instance of horizontal gene transfer (HGT) involving adjacent, discrete β-ketoacyl synthase (KS), acyl carrier protein (ACP) and nonribosomal peptide synthase (NRPS) domains of the epothilone Type I polyketide biosynthetic gene cluster from the myxobacterium Sorangium cellulosom was identified using molecular phylogenetics and sequence analyses. The specific KS domain of the module EPO B fails to cluster phylogenetically with other epothilone KS sequences present at this locus, in contrast to what is typically observed in many other Type I polyketide synthase (PKS) biosynthetic loci. Furthermore, the GC content of the epoB KS, epoA ACP and NRPS domains differs significantly from the base composition of other epothilone domain sequences. In addition, the putatively transferred epothilone loci are located near previously identified transposon-like sequences. Lastly, comparison with other KS loci revealed another possible case of horizontal transfer of secondary metabolite genes in the genus Pseudomonas. This study emphasizes the use of several lines of concordant evidence (phylogenetics, base composition, transposon sequences) to infer the evolutionary history of particular gene and enzyme sequences, and the results support the idea that genes coding for adaptive traits, e.g. defensive natural products, may be prone to transposition between divergent prokaryotic taxa and genomes.


Similar content being viewed by others
References
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–402
August PR, Tang L, Yoon YJ, Ning S, Muller R, Yu TW, Taylor M, Hoffman D, Kim CG, Zhang X (1998) Biosynthesis of the ansamycin antibiotic rifamycin: deductions from the molecular analysis of the rif biosynthetic gene cluster of Amycolatopsis mediterranei S699. Chem Biol 5:69–79
Avise JC, Ball RM (1990) Principles of genealogical concordance in species concepts and biological taxonomy. In: Futuyama D, Antonovics J (eds) Oxford surveys in evolutionary biology, vol 7. Oxford University Press, Oxford, pp 45–67
Bateman A, Birney E, Cerruti L, Durbin R, Etwiller L, Eddy SR, Griffiths-Jones S, Howe KL, Marshall M, Sonnhammer EL (2002) The Pfam protein families database. Nucleic Acids Res 30:276–280
Bentley SD, et al (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Beyer S, Kunze B, Silakowski B, Muller R (1999) Metabolic diversity in myxobacteria—identification of the myxalamid and the stigmatellin biosynthetic gene cluster of Stigmatella aurantiaca Sg a15 and a combined polyketide-(poly) peptide gene cluster from the epothilon producing strain Sorangium cellulosum So ce90. Biochim Biophys Acta 1445:185 −195
Blot M (1994) Transposable elements and adaptation of host bacteria. Genetica 93:5–12
Brown EW, LeClerc JE, Kotewicz ML, Cebula TA (2001) The three R’s of bacterial evolution: how replication, repair, and recombination frame the origin of species. Environ Mol Mutagen 38:248–260
Chadwick DJ, Whelan J (eds) (1992) Secondary metabolites: their function and evolution (Ciba Foundation Symposium). Wiley, Chichester
Charity JC, Pak K, Delwiche CF, Hutcheson SW (2003) Novel exchangeable effector loci associated with the Pseudomonas syringae hrp pathogenicity island: evidence for integron-like assembly from transposed gene cassettes. Mol Plant Microbe Interact 16:495–507
Donadio S, Katz L (1992) Organization of the enzymatic domains in the multifunctional polyketide synthase involved in erythromycin formation in Saccharopolyspora erythraea. Gene 111:51–60
Du L, Shen B (2001) Biosynthesis of hybrid peptide-polyketide natural products. Curr Opin Drug Disc Dev 4:215–228
Du L, Sanchez C, Chen M, Edwards DJ, Shen B (2000) The biosynthetic gene cluster for the antitumor drug bleomycin from Streptomyces verticillus ATCC15003 supporting functional interactions between nonribosomal peptide synthetases and a polyketide synthase. Chem Biol 7:623–642
Eisen J (2000) Horizontal gene transfer among microbial genomes: new insights from complete genome analysis. Curr Opin Genet Dev 10:606–611
Gonnet GH, Benner SA (1991) Computational biochemistry research at ETH. (Technical Report 154, Departement Informatik, Eidgenössische Technische Hochschule, Zürich)
Graur D, Li WH (1997) Fundamentals of molecular evolution. Sinauer, Sunderland, Mass.
Haydock SF, Aparicio JF, Molnar I, Schwecke T, Khaw LE, Konig A, Marsden AF, Galloway IS, Staunton J, Leadlay PF (1995) Divergent sequence motifs correlated with the substrate specificity of (methyl) malonyl-CoA:acyl carrier protein transacylase domains in modular polyketide synthases. FEBS Lett 374:246–248
Henikoff S, Henikoff JG (1992) Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci USA 89:10915–10919
Hopwood DA (1997) Genetic contributions to understanding polyketide synthases. Chem Rev 97:2465–2497
Hopwood DA, Sherman DH (1990) Molecular genetics of polyketides and its comparison to fatty acid biosynthesis. Annu Rev Genet 24:37–66
Huang G, Zhang L, Birch RG (2001) A multifunctional polyketide-peptide synthetase essential for albicidin biosynthesis in Xanthomonas albilineans. Microbiology 147:631–642
Jain R, Rivera MC, Moore JE, Lake JA (2002) Horizontal gene transfer in microbial genome evolution. Theor Popul Biol 61:489–495
Khosla C, Gokhale RS, Jacobsen JR, Cane DE (1999) Tolerance and specificity of polyketide synthases. Annu Rev Biochem 68:219–253
Koonin EV, Makarova KS, Aravind L (2001) Horizontal gene transfer in prokaryotes: quantification and classification. Annu Rev Microbiol 55:709–742
Lan R, Reeves PR (1996) Gene transfer is a major factor in bacterial evolution. Mol Biol Evol 13:47–55
Lawrence JG (1998) Molecular archaeology of the Escherichia coli genome. Proc Natl Acad Sci USA 95:9413–9417
Lawrence JG, Roth JR (1996) Selfish operons: horizontal transfer may drive the evolution of gene clusters. Genetics 143:1843–1860.
Liisa BK, LB, Morton, RA, Golding GB (2001) Codon bias and base composition are poor indicators of horizontally transferred genes. Mol Biol Evol 18:404–412
Longley RE, Gunasekera SP, Faherty D, Mclane J, Dumont F (1993) Immunosuppression by discodermolide. Ann NY Acad Sci 696:94–107
Marahiel MA, Stachelhaus T, Mootz HD (1997) Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem Rev 97:2651–2673
McInerney JO (1998) GCUA: General Codon Usage Analyses. Bioinform Appl Notes 14:372–373
Moffit MC, Neilan BA (2003) Evolutionary affiliations within the superfamily of ketosynthases reflect complex pathway associations. J Mol Evol 56:446–457
Molnar I, et al (2000) The biosynthetic gene cluster for the microtubule-stablizing agents epothilones A and B from Sorangium cellusom So ce90. Chem Biol 7:97–109
Motamedi H, Cai S-J, Shafiee A, Elliston KO (1997) Structural organization of a multifunctional polyketide synthase involved in the biosynthesis of the macrolide immunosuppressant FK506. Eur J Biochem 244:74–80
Nei M, Kumar S (2000) Molecular evolution and phylogenetics. Oxford University Press, Oxford
Ochman H, Lawrence JG, Groisman EA (2000) Lateral gene transfer and the nature of bacterial innovation. Nature 405:299–304
Ohno S (1970) Evolution by gene duplication. Springer-Verlag, New York
Omura S, Ikeda H, Ishikawa J, Hanamoto A, Takahashi C, Shinose M, Takahashi Y, Horikawa H, Nakazawa H, Osonoe T, Kikuchi H, Shiba T, Sakaki Y, Hattori M (2001) Genome sequence of an industrial microorganism Streptomyces avermitilis: deducing the ability of producing secondary metabolites. Proc Natl Acad Sci USA 98:12215–12220
Piel J (2002) A polyketide synthase-peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc Natl Acad Sci USA 99:14002–14007
Romero D, Palacios R (1997) Gene amplification and genomic plasticity in prokaryotes. Annu Rev Genet 31:91–111
Silakowski B, Kunze B, Muller R (2001) Multiple hybrid polyketide synthase/non-ribosomal peptide synthetase gene clusters in the myxobacterium Stigmatella aurantiaca. Gene 275:233–240
Snyder RV, Gibbs PDL, Palacios A, Abiy L, Dickey R, Lopez JV, Rein KS (2003) Polyketide synthase genes from marine dinoflagellates. Marine Biotechnol 5:1–12
Sosio M, Bossi E, Bianci A, Donadio S (2000) Multiple peptide synthetase gene clusters in Actinomycetes. Mol Gen Genet 264:213–221
Staunton J, Weissman KJ. (2001) Polyketide biosynthesis: a millennium review. Natural Prod Rep 18:380–416
Stone MJ, Williams DH (1992) On the evolution of functional secondary metabolites (natural products). Mol Microbiol 6:29–34
Strimmer K, von Haeseler A (1996) Quartet puzzling: a quartet maximum-likelihood method for reconstructing tree topologies. Mol Biol Evol 13:964–969
Sueoka N (1992) Directional mutation pressure, selective constraints, and genetic equilibria. J Mol Evol 34:95–114
Suzuki Y, Gojobori T (1999) A method for detecting positive selection at single amino acid sites. Mol Biol Evol 16:1315–1328
Swofford DL (2001) PAUP*: Phylogenetic Analysis Using Parsimony (*and other methods), Version 4. Sinauer, Sunderland, Mass.
Tang L, Shah S, Chung L, Carney J, Katz L, Khosla C, Julien B (2000) Cloning and heterologous expression of the epothilone gene cluster. Science 287:640–642
Thompson JD, Higgins D, Gibson TJ (1994) CLUSTAL version W: a novel multiple sequence alignment program. Nucleic Acids Res 22:4673–4680.
Tsoi CJ, Khosla C (1995) Combinatorial biosynthesis of ‘unnatural’ natural products: the polyketide example. Chem Biol 2:355–362
Underwood AJ (1998) Experiments in ecology. Cambridge University Press, London
Wagner A (2002) Selection and gene duplication: a view from the genome. Genome Biol 3:1012.1–1012.3
Walton JD (2000) Horizontal gene transfer and the evolution of secondary metabolite gene clusters in fungi: a hypothesis. Fungal Genet Biol 30:167–171
Wiener P, Egan S, Wellington EM (1998) Evidence for transfer of antibiotic-resistance genes in soil populations of streptomycetes. Mol Ecol 7:1205–1216
Zgur-Bertok D (1999) Mechanisms of horizontal gene transfer. Folia Biol (Praha) 45:91–96
Acknowledgements
The author is grateful for critical reading of this manuscript by Drs. Eric Brown and Amy Wright and for statistical analyses by Karen Sandell, M.S. This material is based upon work supported by the National Science Foundation (NSF) under Grant No. 9974984 to JVL, and was carried out in accordance with current laws governing genetic experimentation in the USA. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author and do not necessarily reflect the views of the NSF. This manuscript is Harbor Branch Oceanographic Institution Contribution #1519.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by W. Arber
Rights and permissions
About this article
Cite this article
Lopez, J.V. Naturally mosaic operons for secondary metabolite biosynthesis: variability and putative horizontal transfer of discrete catalytic domains of the epothilone polyketide synthase locus. Mol Genet Genomics 270, 420–431 (2004). https://doi.org/10.1007/s00438-003-0937-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00438-003-0937-9