Abstract
Coccidiosis is a major poultry disease which compromises animal welfare and costs the global chicken industry a huge economic loss. As a result, research entailing coccidial control measures is crucial. Coccidiosis is caused by Eimeria parasites that are highly immunogenic. Consequently, a low dosage of the Eimeria parasite supplied by a vaccine will enable the host organism to develop an innate immune response towards the pathogen. The production of traditional live anticoccidial vaccines is limited by their low reproductive index and high production costs, among other factors. Recombinant vaccines overcome these limitations by eliciting undesired contaminants and prevent the reversal of toxoids back to their original toxigenic form. Recombinant vaccines are produced using defined Eimeria antigens and harmless adjuvants. Thus, studies regarding the identification of potent novel Eimeria antigens which stimulate both cell-mediated and humoral immune responses in chickens are essential. Although the prevalence and risk posed by Eimeria have been well established, there is a dearth of information on genetic and antigenic diversity within the field. Therefore, this paper discusses the potential and efficiency of recombinant vaccines as an anticoccidial control measure. Novel protective Eimeria antigens and their antigenic diversity for the production of cheap, easily accessible recombinant vaccines are also reviewed.
Similar content being viewed by others
Introduction
Coccidiosis is one of the major infectious poultry diseases worldwide. The disease is caused by an obligate intracellular protozoan known as Eimeria (Brown-Jordan et al. 2018). Guven et al. (2013) reported the prevalence of coccidiosis infection to vary between 10% and 90% in industrial chicken farms worldwide. Chickens are economically significant poultry species due to their high protein content, short generation interval, and global acceptability (Blevin et al. 2018; Qi et al. 2018). However, the subclinical and clinical effects of coccidiosis result in malabsorption, reduced feed conversion rate and weight gain, and an increase in mortality and susceptibility to secondary disease in infected chickens (Prakashbabu et al. 2017). Consequently, the livestock industry suffers a global loss exceeding $3 billion dollars, making precautions to control the disease fundamental (Cheng et al. 2018).
Previously, coccidiosis control was administered in the form of anticoccidial drugs, i.e., therapeutic compounds that interrupted the asexual and sexual stages of the parasite (Odden et al. 2018). However, the genetic diversity of Eimeria species enables the parasite to readily develop anticoccidial resistance. In turn, severely limiting their ability to effectively prevent the disease long term (Tan et al. 2017). This leads to the search and discovery of live anticoccidial vaccines which have efficiently treated coccidiosis for more than five decades (Marugan-Hernandez et al. 2016). However, since live vaccines are produced within the chicken itself, it has a low reproductive index of attenuated vaccine pathogens. This increases production costs and limits the production capacity of the vaccine (Blake 2015).
Recent studies have elucidated the potential of recombinant vaccines as an anticoccidial control measure (Clark et al. 2016; Kundu et al. 2017; Lin et al. 2017; Tian et al. 2017). Recombinant vaccines utilize defined antigens cloned from Eimeria species in the presence of an adjuvant or harmless plasmid vector to stimulate immune responses (Suprihati and Yunus 2018). Therefore, various studies are directed at the identification of several novel immune protective genes that could be used to produce recombinant vaccines (Song et al. 2010; Blake et al. 2015; Kundu et al. 2017; Yang et al. 2017; Rafiqi et al. 2018; Suprihati and Yunus 2018). To overcome the naturally occurring genetic diversity of Eimeria, alternative antigens are to be included in the vaccine formulation (Tang et al. 2018a). Current anticoccidial research is targeted at the search and identification of novel protective Eimeria antigens (Yang et al. 2016; Kundu et al. 2017). Therefore, this paper addresses the potential and efficiency of recombinant vaccines as an anticoccidial control measure and identifies novel protective Eimeria antigens, together with their antigenic diversity for the production of cheap, easily accessible recombinant anticoccidial vaccines.
Anticoccidial vaccine potential
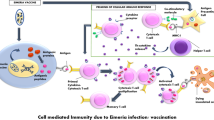
Vaccines provide host organisms with a low dose of a parasite. This, in turn, enables the host organism to develop an innate immunity against subsequent infections of the parasite (Blake and Tomley 2014). Eimeria parasites are highly immunogenic; therefore, a primary infection can effectively stimulate immunity towards subsequent challenges (Muthamilselvan et al. 2016). Hence, in the event of a coccidiosis outbreak in the future, a vaccinated host would display resistance or reduced susceptibility to the disease (Blake and Tomley 2014). In the late 1960s, scientists discovered the potential of using live anticoccidial vaccines to treat coccidiosis (Gadelhaq et al. 2015). The vaccine efficiently treated coccidiosis for over 50 years (Marugan-Hernandez et al. 2016).
Live vaccines are produced from a mixture of non-attenuated and attenuated Eimeria parasites (Hoelzer et al. 2018). Price et al. (2016), Ritzi et al. (2016), and Jenkins et al. (2017) demonstrated the effectiveness and potential of live anticoccidial vaccines in alleviating Eimeria oocyst output in broiler chickens. However, live vaccines are required to be propagated within the chicken itself resulting in a low reproductive index of attenuated vaccine pathogens. This, in turn, increases production costs and limits the efficacy of the vaccine (Tan et al. 2017). Therefore, the need for DNA and recombinant vaccines has become imperative (Pastor-Fernández et al. 2018).
DNA vaccines stimulate cell-mediated immunity by introducing a segment of DNA from the perspective parasite to the host cells. Plasmid vectors introduce naked nucleotide sequences encoding the antigenic portion of the parasite to the host cell, where it is taken up, translated, and the desired protein is expressed (Xu et al. 2008). Xu et al. (2013) reported a 53.7% reduction in oocyst shedding rate coupled with satisfactory immunogenicity and immune protection effects in yellow feathered broilers, treated with a pcDNA3.1 vectored DNA vaccine encoding for the E. maxima Gam56 protein. Panebra and Lillehoj (2019) put forth a similar study displaying effective humoral antibody response, weight gain, and a reduction in broiler chickens challenged with E. acervulina, when treated with an EF-1α-Montanide Gel 01 sequence DNA vaccine. Although, DNA vaccines induce strong long-term cellular immune responses, the vaccine is limited to protein immunogen and risks the possibility of affecting genes which control cell growth (Patra et al. 2017).
Advances in genetic engineering, genomics, molecular biology, and biochemistry provide scientists with novel tools to create potent cost-effective vaccines. Recombinant vaccines overcome the limitations of live and DNA anticoccidial vaccines by eliciting insertional mutagenesis and undesired contaminants and preventing the reversal of toxoids back to their original toxigenic form (Xu et al. 2008; Clark et al. 2017; Sundar et al. 2017; Patra et al. 2017; Barta et al. 2018). Recombinant vaccines work by injecting an immunogenic protein or glycoprotein subunit created in a lab using recombinant DNA technology into the host organism. The vaccine relies on the expression of the proteins as opposed to the DNA itself (Rafiqi et al. 2019; Panebra and Lillehoj 2019). Subunit recombinant vaccines, an essencial category of recombinant vaccines, have drawn much promise over the last few decades (Pastor-Fernández et al. 2018). Subunit vaccines utilize defined antigens cloned from Eimeria species in the presence of an adjuvant or harmless plasmid vector to stimulate both cell-mediated and humoral immune responses (Suprihati and Yunus 2018).
Recombinant subunit anticoccidial vaccines
Subunit vaccines utilize safe antigens such as surface proteins or internal antigens associated with organelles such as microneme, rhoptry, and refractive proteins of sporozoites or merozoites (Clark et al. 2016). These vaccines are produced through the insertion of a gene encoding for an antigen that stimulates an immune response into a vector. Recombinant vaccines or vectored antigens work by inducing incomplete immune protection (Blake and Tomley 2014). This, in turn, reduces selective genetic mutations, which could potentially lead to vaccine resistance and restricts the natural effect of Eimeria recycling. This significantly improves the long-term efficacy of the vaccine (Lin et al. 2017).
Recombinant and natural Eimeriaantigens have successfully stimulated robust immune responses in birds of poultry by establishing a defined host-parasite relationship (Shivaramaiah et al. 2014; Blake et al. 2015; Kundu et al. 2017; Rafiqi et al. 2018). Kundu et al. (2017) reported an increase in IgY and IL-4 sera in chickens vaccinated with a recombinant Immune Mapped protein-1 (EtIMP-1). The author illustrated a 79% reduction in parasite replication compared to the control and chickens vaccinated with live Eimeria oocysts. Tang et al. (2018a) observed partial protection of chickens immunized with an EmIMP1 antigen vaccine against subsequent E. maxima infections. Tian et al. (2017) demonstrated an increase in chicken weight gain, decrease in oocyst outputs, alleviation in the enteric lesions, and induction of moderate anticoccidial index compared to controls in chickens vaccinated with a glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antigen. Song et al. (2010) confirmed the effectiveness of recombinant antigen vaccines using a lactate dehydrogenase (LDH) antigen that decreased oocyst output, duodenal lesions, and body weight loss. Similar results were observed by Rafiqi et al. (2018) with a decrease in caecal lesions when immunized with an SO7 antigen cloned from Eimeria tenella.
Blake et al. (2015) reported that immune protective antigens displayed low levels of polymorphisms, e.g., the low levels of variation were observed in the apical membrane antigen-1 (AMA1) expressed within the E. tenella. Vaccination using a small subset of antigens from a complex Eimeria parasite may provide a significant driving force for immune selection without establishing resistance (Arafat and Abbas 2018). Therefore, current research is directed at the identification of several novel immune protective genes that could be used to produce recombinant vaccines (Song et al. 2010; Blake et al. 2015; Kundu et al. 2017; Yang et al. 2017; Rafiqi et al. 2018; Suprihati and Yunus 2018).
Identification and application of novel antigens
Many studies have been directed towards the identification of novel protective Eimeria antigens (Table 1) (Song et al. 2010; Blake et al. 2015; Kundu et al. 2017; Yang et al. 2017; Rafiqi et al. 2018; Suprihati and Yunus 2018). The identification of immunoprotective genes utilizing expression library immunization has been observed to be an effective tool for novel vaccine development (Arafat and Abbas 2018). Recent studies have suggested that defined antigens such as AMA1, IMP-1, LDH1, and SO7 show potential as successful vaccine candidates (Blake et al. 2015; Lin et al. 2017; Kundu et al. 2017).
Eimeria antigens are identified as potential vaccine candidates based on their host-parasite invasion, interaction, and replication (Suprihati and Yunus 2018). Most of these proteins are secreted within the micronemes organelles situated at the apical tip of the parasite to enable gliding motility attachment and entry and exit from their respective host (Liu et al. 2018). Surface antigens, such as a 25 kDa gene covering 17 and 8 kDa polypeptides, TA4, has been characterized in the early 1990s. Brothers et al. (1998) and Xu et al. (2008) demonstrated the effectiveness of TA4 cloned in a transgenic E. coli against E. tenella. The antigen-produced products which are immunoreactive within a host, subsequently protecting the host against the parasite. Blake et al. (2017) further reviewed the properties and potential of TA4. Rhomboid proteins which aid in Eimeria host invasion has also proven to be an excellent anticoccidial vaccine antigen (Barta et al. 2018). Liu et al. (2013) reported an increase in body weight gain, CD4(+), CD8(+), interleukin-2, and interferon-γ levels, coupled with a decrease in oocyst excretion and cecal lesions in E. tenella challenged chicken broilers when treated with a pVAX1-Rho anticoccidial vaccine. Li et al. (2012) reported similar results brought about by a rhomboid-like gene ETRHO1.
Microneme organelle proteins (MICs) are crucial for parasite host invasion and motility (Barta et al. 2018). MICs are secreted in the early stages of invasion and aid in the attachment of the parasite to the host cells and subsequent formation of the parasite actinomyosin system creating a platform for the invasion (Huang et al. 2018a). Nine MICs have been reported to date, MICs 1–7 and apical membrane antigens (AMA) 1 and 2 (Barta et al. 2018). Huang et al. (2018b) demonstrated a rapid increase in microneme secretion upon host-parasite contact, subsequently blocking host invasion. The authors reported the effective protection against E. mitis induced by EmiMIC3. E. tenella microneme 3 (EtMIC3) and E. tenella microneme 5 (EtMIC5), is due to the antigen binding to sialic acid molecules within the epithelial cell, allowing for cell invasion (Pastor-Fernández et al. 2018). Huang et al. (2018b) identified MIC2, MIC3, and MIC7 of E. maxima; however, the author stated the need for further investigation into their role in host cell attachment. AMA1 proteins, which were primarily extracted from E. maxima and E. tenella, decreased the oocyst output by 66% and 48%, respectively (Blake et al. 2015). Pastor-Fernández et al. (2018) further described the host invasion role of the protein and potential use of the protein isolated from E. tenella to confer a cross-protective vaccine.
Antigen IMP1 induced immune responses against E. maxima in chickens. The antigen contains palmitoylation and myristoylation sites, which confer membrane association proteins (Kundu et al. 2017; Jenkins et al. 2018). Song et al. (2010) illustrated the efficiency of the LDH antigen in chickens infected with coccidiosis. The LDH vaccine stimulated the co-expression of IL-2 and IFN-γ chicken sera. SO7 is a highly immunogenic refractile protein, which is involved in the initial infectious stages of Eimeria. Rafiqi et al. (2018) demonstrated a significant increase in lymphocyte proliferation and levels of IFN-γ and IgY sera in chickens immunized with an E. tenella SO7 (rEtSO7) protein. Hence, illustrating the ability to provide chickens with significant protection against coccidiosis.
Profilin or Eimeria 3-1E is a conserved surface antigen of both merozoites and sporozoites of E. acervulina, E. tenella, and E. maxima. Profilin encodes a 170 aa open reading frame which are expressed in the invasive, sporozoitic, and merozoitic stages of Eimeria (Zhang et al. 2012; Lillehoj et al. 2017). The recombinant protein has successfully induced cell-mediated immunity against live Eimeria species, as illustrated by Tang et al. (2018b) who reported an enhanced protective immunity against E. tenella in birds immunized with Et-EmPro expressing profilin cloned from E. maxima compared to the wild type. Lillehoj et al. (2017) reported similar results with a significant increase in weight gain ratios and decrease in lesion scores in coccidial infected chicken treated with proflin antigens expressed by E. coli compare to the control.
Yang et al. (2017) used a cDNA expression library to identify 6 immune protective genes in chickens. These include four novel genes; EmJS-1, EMHP-1, EMHP-2, and EMRP. The function of EmJS-1 remains unknown. However, EmHP-1 and EmHP-2 code for hypothetical proteins. EmRP is a protein involved in host invasion. Two known proteins, including, EmCKRS and EmSAG, code for a surface antigen glycoprotein that also aids in host invasion (Yang et al. 2017). The genes are located within the region that contains a high antigenic index, such as the T cell epitope motifs. The potential of these genes was demonstrated by the rapid production of antibodies induced when selected chickens were immunized (Liu et al. 2018).
Liu et al. (2017) identified 44 immunodominant proteins among E. acervulina E. tenella, and E. maxima, five of which were identified as common antigens including elongation factors (EF-1 and EF-2), 14-3-3 protein, ubiquitin-conjugating-enzyme-domain-containing protein (UCE), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). EF-1 and EF-2 proteins mediate the translocation of ribosomes in the elongation stage of mRNA translation. Lin et al. (2017) cloned an EF-1α gene from E. tenella, which enabled entry into the host, to generate a vaccine. The vaccine initiated a greater production of EF-1α antibody concentrations post coccidial infection. As a result, cross-protective immunity was induced, resulting in a decrease in the oocyst levels and body weight loss of vaccinated chickens. A similar study was reported by Matsubayashi et al. (2013) who identified the protein produced by the EF-1α gene using a silver-stained PAGE immunoblot.
UCE enzymes are highly conserved ubiquitin-conjugating domains that play a vital role in the biological control of the cells, aiding in protein function, localization, and degradation (Tian et al. 2017). Liu et al. (2017) reported the protection of UCE against E. acervulina, E. tenella, and E. maxima challenged birds. GAPDH is one of the immunogenic common antigens among E. maxima, E. acervulina, and E. tenella. GAPDH vaccinated chickens showed an increase in CD4+ and CD8+ T lymphocytes and IFN-γ, IL-2, IL-4, and IgG antibody levels compared to controls (Tian et al. 2017). Recombinant 14-3-3 proteins are conserved regulatory molecules which aid in cell cycle control, protein localization, mitogenic signal transduction, and apoptotic cell death (Liu et al. 2017). Liu et al. (2018) reported the potential use of Em14-3-3 as an effective vaccine against E. maxima as it drew out both cell-based and humoral immune response. EM14-3-3 is an open reading frame (ORF) of E. maxima that are expressed within the sporozoites and merozoites. The vaccine not only decreased the oocyst output; it increased the body weight gained and CD4+ count but also produced an anticoccidial index that is greater than 165. Liu et al. (2017) further reported the effectiveness of GAPDH and 14-3-3 protein in conferring protection against several species of Eimeria.
Gametocyte antigens including Gam56 and Gam82 from the sexual stages of Eimeria parasites serve as potential vaccine targets in inducing transmission-blocking immunity (Huang et al. 2018b). Sathish et al. (2017) illustrated the ability of E. maxima cloned gametocytes, Gam82 and Gam56, a 82-kDa and 56-kDa tyrosine-rich glycoprotein, respectively, to stimulate immunity against the parasite. The protein is responsible for the oocyst wall formation of E. maxima; hence, the neutralization of the antigens will disrupt the development of Eimeria parasite (Huang et al. 2018b).
The Gam82 and Gam56 proteins induce a high-serum antibody response which in return resulted in a significant reduction in oocyst output and an increased weight gain in vaccinated birds (Sathish et al. 2017). Liu et al. (2014) indicated the important roles in protection against E. maxima infections conferred by EmGAM56, EmGAM82, and EmGAM230. The author further demonstrated the transmission-blocking immunity potential of the recombinant protein EnGAM22. EnGAM22 is a 22-kDa, His- and Pro-rich, intron-free gene directly cloned from E. necatrix. Similar to the EtGAM22 protein, EnGAM22 induces protection against E. tenella and E. maxima-infected chickens (Wiedmer et al. 2017).
Optimal recombinant antigen combinations
A coccidial infection is the result of a combination of several Eimeria species, each of which may differ significantly in terms of their morphology and physiology (Brown-Jordan et al. 2018). Therefore, recent studies have elucidated the need to combine multiple Eimeria antigens from respective Eimeria spp. into a single formulation (Blake et al. 2017). This led to the construction of multivalent epitope DNA vaccines which are able to treat a number of species with an infection (Pastor-Fernández et al. 2018). Multivalent DNA vaccines are able to induce cellular immunity against a series of Eimeria species (Meunier et al. 2016). Song et al. (2015) reported the alleviation of enteric lesions and oocyst output, coupled with an increase in body weight gain in E. acervuline, E. tenella, E. maxima, and E. necatrix challenged birds, when treated with pVAX1-NA4–1-TA4–1-LDH-2-EMCDPK-1-IL-2 and pVAX1-NA4–1-TA4–1-LDH-2-EMCDPK-1 multivalent DNA vaccines. Tian et al. (2017) reported similar results on the efficiency of a multivalent pET-32a vectored anticoccidial vaccines containing EaGAPDH and EmGAPDH genes in Eimeria challenged birds.
Wang et al. (2014) outlined the successful combination of a cytokine chicken IL-2 and rhomboid-like gene of E. tenella in the formation of a Bacille Calmette-Guerin (rBCG) recombination vaccine. Xu et al. (2008) reported the enhanced induced immunity against Eimeria challenged chickens when exposed to a TA4 and chIL-2 cocktail vaccine. Transgenic Eimeria species serve as a powerful tool in the development of multivalent anticoccidial vaccine by generating parasite lines which express antigens that target multiple Eimeria species (Pastor-Fernández et al. 2018), However, optimal antigen combination identification depends on the assessment of pre-existing antigenic diversity within each identified antigen (Pastor-Fernández et al. 2018).
Diversity of Eimeria antigens
The natural genetic polymorphism and complex life cycle of Eimeria species make the formulation of recombinant vaccines a difficult task (Pastor-Fernández et al. 2018). To compensate for the naturally occurring genetic diversity of Eimeria, alternative antigens are included in the vaccine formula. This is enabled by each Eimeria species producing between 6000 and 9000 antigens (Reid et al. 2014). Antigenic diversity occurs in two forms (Smith et al. 2002). The first involves antigenic diversity within the parasite; therefore, an infection in one subpopulation will not induce cross-protection in another subpopulation challenged with Eimeria (Suprihati and Yunus 2018). The second type is produced when variant proteins that are encoded by large polymorphic gene families are exchanged allowing the parasite to persist in a single host under an immune system attack (Pastor-Fernández et al. 2018).
Kundu et al. (2017) reported low nucleotide diversity within the IMP-1 gene in E. tenella across four countries, China, India, UK, and the USA. The source of the diversity lies within the contraction and expansion of five substitutions and a CAG triplet repeat. Blake et al. (2015) showed a low level of balancing selection within the AMA1 region of E. tenella. The author attributed the lack of diversity to parasite interbreeding and absence of migration. However, DNA-based sequence-led studies of monoclonal Eimeria populations in Northern and Southern India indicated high levels of genomic diversity within the AMA1 gene. The diversity resulted from allopatric diversification between Southern and Northern India, in which there were 98% and 87.5% haplotypes unique in these regions, respectively (Vrba and Pakandl 2014). Eimeria haplotypes have a low occurrence rate in Northern and Southern India and Nigeria due to reduced cross-fertilization (Clark et al. 2017). Clark et al. (2016) reported a correlated increase in haplotype diversity and increased distances between the sampling areas.
Although many antigens were proposed for recombinant vaccines in order to reduce the severity of the disease, lessen replication potential of the parasite, improve feed conversion, and increase body weight, delivery of protective antigens in a scalable, effective, and affordable manner remains one of the biggest challenges in vaccine development (Hoelzer et al. 2018; Pastor-Fernández et al. 2018).
Enhancement of protective antigen activity
Adjuvants are substances which enhance induced immunity (Li et al. 2018). Montanide IMS 106 and IMS 101 adjuvants are a dispersion of liquid nanoparticles which enhance immune responses and are compatible with various buffered antigens (Jang et al. 2012). Lillehoj et al. (2017) demonstrated the potential of recombinant purified profilin and NetB proteins mixed with IMS adjuvants in combating coccidiosis. Chickens treated with the adjuvants exhibited significantly increased body weight gains in coccidial-infected group compared to the group treated with profilin only and the controls. Rafiqi et al. (2018) reported similar results in broiler chickens immunized with rEtSO7 antigen adjuvant with Montanide ISA 71 VG. In addition to increased body weight gains, the adjuvant vaccines showed a reduction in lesion scores and oocyst outputs compared to the controls. Zhang et al. (2012) demonstrated enhanced efficiency of recombinant vaccines with an adjuvant, ginsenosides. Ginsenosides are extracted from the root of a Ginseng plant and are able to stimulate both humoral and cellular immune responses against a variety of infections (Dkhil and Al-Quraishy 2016; Li et al. 2018). Whereby the profilin-specific antibody level and body weight gain was significantly higher in groups vaccinated with proflin and ginsome compared to the groups treated solely with profiling and the controls (Zhang et al. 2012).
Delivery of protective antigens
Recombinant vaccine development requires an effective adjuvant or delivery system which distributes the antigen to the respective site in adequate quantities to initiate an immune response (Bottje et al. 2018). Delivery of the epitopes to its respective targets is the most important consideration in recombinant development as the epitope enables locomotion and the invasion of the vector (Sundar et al. 2017). A number of vectors have been reported including eukaryotic expression vectors such as Salmonella strains, pVAX1 vectors, yeasts such as Saccharomyces cerevisiae, pMV361, with several showing promise (Song et al. 2010; Sun et al. 2014; Bottje et al. 2018).
Salmonella strains make suitable vectors as the bacterial genes may be mutated or attenuated to create bacteria with low to no pathogenesis to the infected or immunized subject while maintaining immunogenicity. Salmonella can survive the gastrointestinal tract of the host and give rise to a mucosal immune response. Bottje et al. (2018) utilized Salmonella as a vector to deliver an E. maxima antigen, EmTFP250, to hens. Song et al. (2010) created an LDH antigen recombinant vaccine using a eukaryotic expression vector pVAX1 to prevent E. acervulina. Sun et al. (2014) utilized an S. cerevisiae vector containing an EtMic2 microneme protein to stimulate an immune response in chickens. This, in turn, reduced oocyst output and cecal pathology while increasing body weight gain. The pMV361 vector is used in the production of the rBCG pMV361-rho and pMV361-rho-IL2 vaccines which effectively protects chicken against E. tenella by alleviating oocyst output and caecal lesions (Wang et al. 2014). Li et al. (2018) reported similar results generated by an EtAMA1- Lactococcus lactis recombinant vaccine.
Marugan-Hernandez et al. (2016) demonstrated the potential of using Eimeria parasites as vectors for various other pathogenic poultry infections. The large size of Eimeria genomes, together with their ability to induce a range of immune responses in hosts, makes the parasites an attractive vector in the development of multivalent recombinant vaccines. Tang et al. (2018a) described transgenic E. tenella as a vector, which expressed the EmIMP1 antigen of E. maxima. Clark et al. (2012) reported the use of an E. tenella vaccine, which expressed the Campylobacter jejuni antigen (CjaA). Upon immunization, the incidence of campylobacteriosis was 90% lower in test subjects compared to controls. Liu et al. (2018) indicated the generation of antibiotics against viral proteins. For example, transgenic strains of Eimeria were able to express the Matrix-2 (M2) protein that is expressed by an avian influenza virus. The expressed antigen was able to initiate an immune response.
Marugan-Hernandez et al. (2016) utilized transgenic sporozoites of E. tenella to express antigens capable of inducing protection against two economically significant poultry diseases, the infectious bursal disease virus (IBDV) and the infectious laryngotracheitis virus (ILTV). Two sets of antigens, gI, and vvVP2, and ILTV and IBDV, respectively, were cloned into plasmid constructs containing the mCherry fluorophore reporter protein. The results demonstrated that foreign antigens were expressed by E. tenella and were readily available to the host’s immune system. Blake and Tomley (2014) and Lillehoj et al. (2018) demonstrated the potential of viral and plasmid vectors in the development of anticoccidial vaccines, respectively. Blake and Tomley (2014) demonstrated the effectiveness of the herpes simplex virus (HSV) and Fowlfox virus (FWPV) as vectors for the delivery of lactate dehydrogenase and E. acervulina refractile proteins. Lillehoj et al. (2018) reported protective immunity in E. tenella challenged chickens immunized with pET-EF10, an EF-la antigen cloned in a pET32a + plasmid vector.
Shivaramaiah et al. (2014) proposed the use of plants as an edible, less intrusive vaccine source against coccidiosis. Plants can be genetically engineered to express parasitic antigens in order to initiate immune responses. Nicotiana tabacum modified to express EtMIC1 and EtMIC2 of E. tenella as polyhistidine tagged fusion proteins indicated a reduction in oocyst output together with a high antibody production in birds fed with the plant (Jacob et al. 2013). Sathish et al. (2017) reported a 39% increase in weight gain coupled with a 69% reduction in oocyst output in birds immunized with a transgenic Tabaco plant that expressed the Gam82 protein. Plant-based vaccine technology is relatively cost-effective as the vaccine enables poultry farmers to dose stable vaccines with feed that allow for both mucosal and systemic protection (Aswathi et al. 2014).
Conclusion
Recombinant vaccines use a small subset of Eimeria antigens to provide a significant driving force for immune selection without establishing resistance. This review summarized a number of novel antigens that could be used to generate potent recombinant anticoccidial vaccines. These antigens include MICs, IMP1, LDH, rEtSO7, EmJS-1, EMHP-1, EMHP-2, EMRP, EmCKRS, EmSAG, EF-1α, GAPDH, Eimeria gametocyte antigens, TA4 and Em14-3-3. The formulation of recombinant vaccines is quite challenging due to the genetic polymorphism and complex life cycle of Eimeria species. Therefore, it is important to consider that haplotype diversity increases with an increase in distance between sampling areas. Notably, haplotype diversity can be reduced through cross-fertilization. Significant progress has been made in the delivery of protective antigens to their respective locations in a scalable, effective, and affordable manner. A number of eukaryotic expression vectors, including pVAX1 vector, Salmonella strains, and yeasts, together with the Eimeria parasite itself, have shown the potential to efficiently deliver antigens to the respective site in adequate quantities to initiate an immune response. Recent studies have elucidated the use of edible vaccines and inclusion of adjuvants which hold great promise for the development of cost-effective efficient anticoccidial vaccines. Coccidiosis remains a major infectious disease in poultry industry globally with a huge economic loss; however, application of genomics is providing an improvement in diagnosis and control of the disease.
References
Al-Idreesi SR, Kweider M, Katranji MM (2013) Immune responses to Eimeria tenella sporozoite protein as vaccine to broiler against Coccidiosis. Int J Poult Sci 12:582–591
Arafat N, Abbas I (2018) Coccidia of Japanese quail: from identification, prevalence, infection, and immunization. J Parasitol 104:23–30
Aswathi PB, Bhanja SK, Yadav AS, Rekha V, John JK, Gopinath D, Sadanandan GV, Shinde A, Jacob A (2014) Plant based edible vaccines against poultry diseases?: a review. Adv Anim Vet Sci 2:305–311
Barta JR, Berghman L, Shivaramaiah S, Faulkner OB, Bielke L, Hargis B (2018) Compositions and methods of enhancing immune responses to Eimeria or limiting Eimeria infection. United States patent US 9,884,099 https://patents.google.com/patent/US9884099B2/en Accessed 28 Dec 2018
Blake DP (2015) Eimeria genomics: where are we now and where are we going? Vet Parasitol 212:68–74
Blake DP, Tomley FM (2014) Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol 30:12–19
Blake DP, Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Jatau ID, Ayoade S, Kawahara F, Moftah A, Reid AJ, Adebambo AO, Alvarez-Zapata R, Srinivasa-Rao AS, Thangaraj K, Banerjee PS, Dhinakar-Raj G, Raman M, Tomley FM (2015) Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proc Natl Acad Sci U S A 112:5343–5350
Blake DP, Pastor-Fernández I, Nolan MJ, Tomley FM (2017) Recombinant anticoccidial vaccines-a cup half full? Infect Genet Evol 55:358–365
Blevin RE, Kim SA, Park SH, Rivera R, Ricke SC (2018) Historical, current, and future prospects for food safety in poultry product processing systems. In Food and Feed Safety Systems and Analysis. Academic Press, London, 323–345
Bottje W, Hargis B, Berghman L, Kwon YM, Cole K, Cox M, Layton S, El-Ashram S, Barta J, Tellez G (2018) Compositions and methods of enhancing immune responses to Eimeria. U.S. Patent Application. https://patents.google.com/patent/US10016493B2/en Accessed on 28 Dec 2018
Brothers VM, Kuhn I, Paul LS, Gabe JD, Andrews WH, Sias SR, McCaman MT, Dragon EA, Files JG (1998) Characterization of a surface antigen of Eimeria tenella sporozoites and synthesis from a cloned cDNA in Escherichia coli. Mol Biochem Parasitol 28:235–247
Brown-Jordan A, Blake D, Beard J, Beharry A, Serrette L, Soleyn A, Oura C (2018) Molecular identification of Eimeria species in broiler chickens in Trinidad, West Indies. Vet Sci 5:12–21
Cheng P, Wang C, Lin X, Zhang L, Fei C, Zhang K, Wang X (2018) Pharmacokinetics of a novel triazine ethanamizuril in rats and broiler chickens. Res Vet Sci 117:99–103
Clark JD, Oakes RD, Redhead K, Crouch CF, Francis MJ, Tomley FM, Blake DP (2012) Eimeria species parasites as novel vaccine delivery vectors: anti-Campylobacter jejuni protective immunity induced by Eimeria tenella delivered CjaA. Vaccine 30:2683–2688
Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Kumar S, Ayoade S, Fornace KM, Jatau ID, Moftah A, Nolan MJ (2016) Cryptic Eimeria genotypes are common across the southern but not northern hemisphere. Int J Parasitol 46:537–544
Clark EL, Tomley FM, Blake DP (2017) Are Eimeria genetically diverse, and does it matter? Trends Parasitol 33:231–241
Dkhil MA, Al-Quraishy S (2016) Nanoparticles against Eimeriosis. InNanoparticles in the fight against parasites. Springer, Cham. 1; 207–210
Gadelhaq SM, Arafa WM, Aboelhadid SM (2015) Molecular characterization of Eimeria species naturally infecting Egyptian Baldi chickens. Iran J Parasitol 10:87–95
Guven E, Beckstead RB, Kar S, Vatansever Z, Karaer Z (2013) Molecular identification of Eimeria species of broiler chickens in Turkey. Ank Univ Vet Fak Derg 60:245–250
Hoelzer K, Bielke L, Blake DP, Cox E, Cutting SM, Devriendt B, Erlacher-Vindel E, Goossens E, Karaca K, Lemiere S, Metzner M (2018) Vaccines as alternatives to antibiotics for food producing animals. Part 2: new approaches and potential solutions. Vet Res 49:70–82
Huang J, Liu T, Li K, Song X, Yan R, Xu L, Li X (2018a) Proteomic analysis of protein interactions between Eimeria maxima sporozoites and chicken jejunal epithelial cells by shotgun LC-MS/MS. Parasit Vectors 11:226–230
Huang X, Liu J, Tian D, Li W, Zhou Z, Huang J, Song X, Xu L, Yan R, Li X (2018b) The molecular characterization and protective efficacy of microneme 3 of Eimeria mitis in chickens. Vet Parasitol 258:114–123
Jacob SS, Cherian S, Sumithra TG, Raina OK, Sankar M (2013) Edible vaccines against veterinary parasitic diseases – current status and future prospects. Vaccine 31:1879–1885
Jang SI, Lillehoj HS, Lee SH, Lee KW, Park MS, Cha SR, Lillehoj EP, Subramanian BM, Sriraman R, Srinivasan VA (2010) Eimeria maxima recombinant Gam82 gametocyte antigen vaccine protects against coccidiosis and augments humoral and cell-mediated immunity. Vaccine 28:2980–2985
Jang SI, Lillehoj HS, Lee SH, Lee KW, Lillehoj EP, Hong YH, An DJ, Jeong W, Chun JE, Bertrand F, Dupuis L, Deville S, Arous JB (2012) Vaccination with Clostridium perfringens recombinant proteins in combination with Montanide™ ISA 71 VG adjuvant increases protection against experimental necrotic enteritis in commercial broiler chickens. Vaccine 30:5401–5406
Jenkins MC, Parker C, Ritter D (2017) Eimeria oocyst concentrations and species composition in litter from commercial broiler farms during anticoccidial drug or live Eimeria oocyst vaccine control programs. Avian Dis 61:214–220
Jenkins MC, Stevens L, O'Brien C, Parker C, Miska K, Konjufca V (2018) Incorporation of a recombinant Eimeria maxima IMP1 antigen into nanoparticles confers protective immunity against E. Maxima challenge infection. Vaccine 36:1126–1131
Klotz C, Gehre F, Lucius R, Pogonka T (2007) Identification of Eimeria tenella genes encoding for secretory proteins and evaluation of candidates by DNA immunisation studies in chickens. Vaccine 25:6625–6634
Kundu K, Garg R, Kumar S, Mandal M, Tomley FM, Blake DP, Banerjee PS (2017) Humoral and cytokine response elicited during immunisation with recombinant immune mapped protein-1 (EtIMP-1) and oocysts of Eimeria tenella. Vet Parasitol 244:44–53
Li JH, Huang XS, Zhang GC (2010) Immune response and protective efficacy against homologous challenge in BALB/c mice vaccinate with DNA vaccine encoding Toxoplasma gondiiactin depolymerizing factor gene. Vet Parasitol 179:1–6
Li J, Zheng J, Gong P, Zhang X (2012) Efficacy of Eimeria tenella rhomboid-like protein as a subunit vaccine in protective immunity against homologous challenge. Parasitol Res 110:1139–1145
Li WC, Zhang XK, Du L, Pan L, Gong PT, Li JH, Yang J, Li H, Zhang XC (2013) Eimeria maxima: efficacy of recombinant Mycobacterium bovis BCG expressing apical membrane antigen1 against homologous infection. Parasitol Res 112:3825–3833
Li J, Wang F, Ma C, Huang Y, Wang D, Ma D (2018) Recombinant lactococcus lactis expressing Eimeria tenella AMA1 protein and its immunological effects against homologous challenge. Exp Parasitol 191:1–8
Lillehoj HS, Jang SI, Panebra A, Lillehoj EP, Dupuis L, Arous JB, Lee SK, Oh ST (2017) In ovo vaccination using Eimeria profilin and Clostridium perfringens NetB proteins in Montanide IMS adjuvant increases protective immunity against experimentally-induced necrotic enteritis. Asian Australas J Anim Sci 30:1478–1489
Lillehoj HS, Sungtaek OH, Panebra A (2018) Eimeria tenella elongation factor-1 alpha recombinant immunogenic compositions which induce active protective immunity against avian coccidiosis. United States patent application US 15/887,130. https://patents.google.com/patent/US20180230475A1/en Accessed 3 Jan 2019
Lin RQ, Lillehoj HS, Lee SK, Oh S, Panebra A, Lillehoj EP (2017) Vaccination with Eimeria tenella elongation factor-1α recombinant protein induces protective immunity against E. tenella and E. maxima infections. Vet Parasitol 243:79–84
Liu Y, Zheng J, Li J, Gong P, Zhang X (2013) Protective immunity induced by a DNA vaccine encoding Eimeria tenella rhomboid against homologous challenge. Parasitol Res 112:251–257
Liu D, Cao L, Zhu Y, Deng C, Su S, Xu J, Jin W, Li J, Wu L, Tao J (2014) Cloning and characterization of an Eimeria necatrix gene encoding a gametocyte protein and associated with oocyst wall formation. Parasit Vectors 7:27–35
Liu L, Huang X, Liu J, Li W, Ji Y, Tian D, Tian L, Yang X, Xu L, Yan R, Li X (2017) Identification of common immunodominant antigens of Eimeria tenella, Eimeria acervulina and Eimeria maxima by immunoproteomic analysis. Oncotarget 8:34935–34941
Liu T, Huang J, Ehsan M, Wang S, Hong F, Zhou Z, Li X (2018) Protective immunity against Eimeria maxima induced by vaccines of Em14-3-3 antigen. Vet Parasitol 251:241–249
Marugan-Hernandez V, Cockle C, Macdonald S, Pegg E, Crouch C, Blake DP, Tomley FM (2016) Viral proteins expressed in the protozoan parasite Eimeria tenella are detected by the chicken immune system. Parasit Vectors 9:463–478
Matsubayashi M, Teramoto-Kimata I, Uni S, Lillehoj HS, Matsuda H, Furuya M, Sasai K (2013) Elongation factor-1α is a novel protein associated with host cell invasion and a potential protective antigen of Cryptosporidium parvum. J Biol Chem 288:34111–34120
Meunier M, Chemaly M, Dory D (2016) DNA vaccination of poultry: the current status in 2015. Vaccine 34:202–211
Muthamilselvan T, Kuo TF, Wu YC, Yang WC (2016) Herbal remedies for coccidiosis control: a review of plants, compounds, and anticoccidial actions. eCAM 2016:301–311
Odden A, Denwood MJ, Stuen S, Robertson LJ, Ruiz A, Hamnes IS, Enemark HL (2018) Field evaluation of anticoccidial efficacy: a novel approach demonstrates reduced efficacy of toltrazuril against ovine Eimeria spp. in Norway. Int J Parasitol Drugs Drug Resist 8:304–311
Panebra A, Lillehoj HS (2019) Eimeria tenella elongation factor-1α (EF-1α) co-administered with chicken IL-7 (chIL-7) DNA vaccine emulsified in Montanide gel 01 adjuvant enhanced the immune response to E. acervulina infection in broiler chickens. Avian Dis 63:145–152
Pastor-Fernández I, Kim S, Billington K, Bumstead J, Marugán-Hernández V, Küster T, Tomley FM (2018) Development of cross-protective Eimeria-vectored vaccines based on apical membrane antigens. Int J Parasitol 48:505–518
Patra G, Kumar A, Ghosh S, Lalnunpuia C, Bachan M, Saikia B, Bhagawati J (2017) Vaccines against protozoan parasites of veterinary importance: a review. J Entomol Zool Stud 5:1016–1021
Prakashbabu BC, Thenmozhi V, Limon G, Kundu K, Kumar S, Garg R, Clark EL, Rao AS, Raj DG, Raman M, Banerjee PS (2017) Eimeria species occurrence varies between geographic regions and poultry production systems and may influence parasite genetic diversity. Vet Parasitol 233:62–72
Price KR, Hafeez MA, Bulfon J, Barta JR (2016) Live Eimeria vaccination success in the face of artificial non-uniform vaccine administration in conventionally reared pullets. Avian Pathol 45:82–93
Qi X, Fu Y, Wang RY, Ng CN, Dang H, He Y (2018) Improving the sustainability of agricultural land use: an integrated framework for the conflict between food security and environmental deterioration. Appl Geogr 90:214–223
Rafiqi SI, Garg R, Reena KK, Ram H, Singh M, Banerjee PS (2018) Immune response and protective efficacy of Eimeria tenella recombinant refractile body protein, EtSO7, in chickens. Vet Parasitol https://www.sciencedirect.com/science/article/pii/S0304401718302334 Accessed 28 Dec 2018 258:108–113
Rafiqi SI, Garg R, Ram H, Reena KK, Asari M, Kumari P, Kundave VR, Singh M, Banerjee PS (2019) Immunoprophylactic evaluation of recombinant gametocyte 22 antigen of Eimeria tenella in broiler chickens. Parasitol Res 118:945–953
Reid AJ, Blake DP, Ansari HR, Billington K, Browne HP, Bryant JM, Dunn M, Hung SS, Kawahara F, Miranda-Saavedra D, Malas T (2014) Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res 24:1676–1685
Ritzi MM, Abdelrahman W, Van-Heerden K, Mohnl M, Barrett NW, Dalloul RA (2016) Combination of probiotics and coccidiosis vaccine enhances protection against an Eimeria challenge. Vet Res 47:111–121
Sathish K, Sriraman R, Subramanian BM, Rao NH, Balaji K, Narasu ML, Srinivasan VA (2011) Plant expressed EtMIC2 is an effective immunogen in conferring protection against chicken coccidiosis. Vaccine 29:9201–9208
Sathish K, Mohana S, Bala MS, Harini C, Ponanna NM, Srinivasan VA, Rajan S (2017) Subunit vaccine based on plant expressed recombinant Eimeria gametocyte antigen Gam82 elicit protective immune response against chicken coccidiosis. J Vaccines 8:6–13
Shivaramaiah C, Barta J, Hernandez-Velasco X, Téllez G, Hargis B (2014) Coccidiosis: recent advancements in the immunobiology of Eimeria species, preventive measures, and the importance of vaccination as a control tool against these apicomplexan parasites. Dove Press https://www.dovepress.com/coccidiosis-recent-advancements-in-the-immunobiology-of-eimeria-specie-peer-reviewed-fulltext-article-VMRR#ref27 Accessed 12 Jan 2019
Smith AL, Hesketh P, Archer A, Shirley MW (2002) Antigenic diversity in Eimeria maxima and the influence of host genetics and immunization schedule on cross-protective immunity. Infect Immun 70:2472–2479
Song H, Yan R, Xu L, Song X, Shah MAA, Zhu H, Li X (2010) Efficacy of DNA vaccines carrying Eimeria acervulina lactate dehydrogenase antigen gene against coccidiosis. Exp Parasitol 126:224–231
Song X, Ren Z, Yan R, Xu L, Li X (2015) Induction of protective immunity against Eimeria tenella, Eimeria necatrix, Eimeria maxima and Eimeria acervulina infections using multivalent epitope DNA vaccines. Vaccine 33:2764–2770
Sun H, Wang L, Wang T, Zhang J, Liu Q, Chen P, Zhao X (2014) Display of Eimeria tenella EtMic2 protein on the surface of Saccharomyces cerevisiae as a potential oral vaccine against chicken coccidiosis. Vaccine 32:1869–1876
Sundar STB, Harikrishnan TJ, Latha BR, Chandra GS, Kumar TMSA (2017) Anticoccidial drug resistance in chicken coccidiosis and promising solutions: a review. J Entomol Zool Stud 5:1526–1529
Suprihati E, Yunus M (2018) Evaluation of the antigenicity and immunogenicity of Eimeria tenella by reproductive index and histopathological changes of cecal coccidiosis virulent live vaccine in broiler chickens. Afr J Infect Dis 12:104–110
Tan L, Li Y, Yang X, Ke Q, Lei W, Mughal MN, Zhao J (2017) Genetic diversity and drug sensitivity studies on Eimeria tenella field isolates from Hubei Province of China. Parasit Vectors 10:137–149
Tang X, Liu X, Yin G, Suo J, Tao G, Zhang S, Suo X (2018a) A novel vaccine delivery model of the apicomplexan Eimeria tenella expressing Eimeria maxima antigen protects chickens against infection of the two parasites. Front Immunol 8:1982–1994
Tang X, Suo J, Li C, Du M, Wang C, Hu D, Duan C, Lyu Y, Liu X, Suo X (2018b) Transgenic Eimeria tenella expressing profilin of Eimeria maxima elicits enhanced protective immunity and alters gut microbiome of chickens. Infect Immun 86:00808–00817
Tian L, Li W, Huang X, Tian D, Liu J, Yang X, Song X (2017) Protective efficacy of coccidial common antigen glyceraldehyde 3-phosphate dehydrogenase (GAPDH) against challenge with three Eimeria species. Front Microbiol 8:124–132
Vrba V, Pakandl M (2014) Coccidia of turkey: from isolation, characterisation and comparison to molecular phylogeny and molecular diagnostics. Int J Parasitol 44:985–1000
Wang Q, Chen L, Li J, Zheng J, Cai N, Gong P, Li S, Li H, Zhang X (2014) A novel recombinant BCG vaccine encoding Eimeria tenella rhomboid and chicken IL-2 induces protective immunity against coccidiosis. Korean J Parasitol 52:251–258
Wiedmer S, Erdbeer A, Volke B, Randel S, Kapplusch F, Hanig S, Kurth M (2017) Identification and analysis of Eimeria nieschulzi gametocyte genes reveal splicing events of gam genes and conserved motifs in the wall-forming proteins within the genus Eimeria (Coccidia, Apicomplexa). Parasite 2017:24–30
Xu Q, Song X, Xu L, Yan R, Shah MA, Li X (2008) Vaccination of chickens with a chimeric DNA vaccine encoding Eimeria tenella TA4 and chicken IL-2 induces protective immunity against coccidiosis. Vet Parasitol 156:319–323
Xu J, Zhang Y, Tao J (2013) Efficacy of a DNA vaccine carrying Eimeria maxima Gam56 antigen gene against coccidiosis in chickens. Korean J parasitol 51:147–156
Yan M, Cui X, Zhao Q, Zhu S, Huang B, Wang L, Zhao H, Liu G, Li Z, Han H, Dong H (2018) Molecular characterization and protective efficacy of the microneme 2 protein from Eimeria tenella. Parasite 25:153–164
Yang R, Brice B, Ryan U (2016) Morphological and molecular characterization of Eimeria purpureicephali n. sp. (Apicomplexa: Eimeriidae) in a red-capped parrot (Purpureicephalus spurius, Kuhl, 1820) in Western Australia. Int J Parasitol Parasites Wildl 5:34–39
Yang G, Yao J, Yang W, Jiang Y, Du J, Huang H, Gu W, Hu J, Ye L, Shi C, Shan B (2017) Construction and immunological evaluation of recombinant lactobacillus plantarum expressing SO7 of Eimeria tenella fusion DC-targeting peptide. Vet Parasitol 236:7–13
Zhang DF, Xu H, Sun BB, Li JQ, Zhou QJ, Zhang HL, Du AF (2012) Adjuvant effect of ginsenoside-based nanoparticles (ginsomes) on the recombinant vaccine against Eimeria tenella in chickens. Parasitol Res 110:2445–2453
Zhao YL, Bao YZ, Zhang LJ, Chang LY, Jiang LF, Liu YW, Zhang L, Qin JH (2013) Biosafety of the plasmid pcDNA3-1E of Eimeria acervulina in chicken. Exp Parasitol 133:231–236
Zhao Y, Xu R, Zhang Y, Ji X, Zhang J, Liu Y, Bao Y, Qin J (2014) Protective efficacy in chickens of recombinant plasmid pET32a (+)-ADF-3-1E of Eimeria acervulina. Parasitol Res 113:3007–3014
Funding
The National Research Foundation (NRF) provided financial support (Grant numbers: 112768 and 116935).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Section Editor: Berit Bangoura
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Venkatas, J., Adeleke, M.A. A review of Eimeria antigen identification for the development of novel anticoccidial vaccines. Parasitol Res 118, 1701–1710 (2019). https://doi.org/10.1007/s00436-019-06338-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-019-06338-2