Abstract
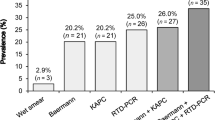
Human infection with the nematode Strongyloides stercoralis, which may have a life-threatening course, primarily occurs in tropical settings. Epidemiological data on the occurrence of strongyloidiasis are scarce, and microscopic stool-based detection methods are insensitive. Polymerase chain reaction (PCR) assays have been developed, yet conflicting results have been reported. Our goal was to determine whether there was diagnostic agreement between an in-house PCR and two microscopic techniques, the Baermann funnel (BM) and the Koga agar plate culture (KAP) for the detection of S. stercoralis in stool samples. Eighty ethanol-fixed stool samples stemming from a cross-sectional survey in Maluku, Indonesia, were purposefully selected for PCR analysis. The final sample size comprised four groups, each with 20 samples: group 1, positive for S. stercoralis on both BM and KAP; group 2, positive only by BM; group 3, positive only by KAP; and group 4, negative on both BM and KAP. A Strongyloides-specific PCR targeting the internal transcribed spacer 2 (ITS2) region was carried out in an Indonesian reference laboratory. The overall agreement between PCR and microscopy was 61% (49/80 samples), being highest in group 1 (15/20, 75%) and lowest in group 3 (9/20, 45%). PCR revealed eight additional S. stercoralis infections in group 4. Future studies should elucidate the ‘true’ infection status of samples that are negative by PCR, but positive upon microscopy. Taken together, there is a lack of agreement between microscopy and PCR results for the diagnosis of human S. stercoralis infection in Indonesia. ClinicalTrials.gov (identifier: NCT02105714)


Similar content being viewed by others
References
Albonico M, Becker SL, Odermatt P, Angheben A, Anselmi M, Amor A, Barda B, Buonfrate D, Cooper P, Gétaz L, Keiser J, Khieu V, Montresor A, Muñoz J, Requena-Méndez A, Savioli L, Speare R, Steinmann P, van Lieshout L, Utzinger J, Bisoffi Z, StrongNet Working Group (2016) StrongNet: an international network to improve diagnostics and access to treatment for strongyloidiasis control. PLoS Negl Trop Dis 10:e0004898. https://doi.org/10.1371/journal.pntd.0004898
Alirol E, Horie NS, Barbé B, Lejon V, Verdonck K, Gillet P, Jacobs J, Büscher P, Kanal B, Bhattarai NR, El Safi S, Phe T, Lim K, Leng L, Lutumba P, Mukendi D, Bottieau E, Boelaert M, Rijal S, Chappuis F (2016) Diagnosis of persistent fever in the tropics: set of standard operating procedures used in the NIDIAG febrile syndrome study. PLoS Negl Trop Dis 10:e0004749. https://doi.org/10.1371/journal.pntd.0004749
Amor A, Rodriguez E, Saugar JM, Arroyo A, Lopez-Quintana B, Abera B, Yimer M, Yizengaw E, Zewdie D, Ayehubizu Z, Hailu T, Mulu W, Echazú A, Krolewieki AJ, Aparicio P, Herrador Z, Anegagrie M, Benito A (2016) High prevalence of Strongyloides stercoralis in school-aged children in a rural highland of north-western Ethiopia: the role of intensive diagnostic work-up. Parasit Vectors 9:617. https://doi.org/10.1186/s13071-016-1912-8
Barda B, Wampfler R, Sayasone S, Phongluxa K, Xayavong S, Keoduangsy K, Schindler C, Keiser J (2018) Evaluation of two DNA extraction methods on the detection of Strongyloides stercoralis infection. J Clin Microbiol 56:e01941–e01917. https://doi.org/10.1128/JCM.01941-17
Becker SL, Sieto B, Silué KD, Adjossan L, Koné S, Hatz C, Kern WV, N’Goran EK, Utzinger J (2011) Diagnosis, clinical features, and self-reported morbidity of Strongyloides stercoralis and hookworm infection in a co-endemic setting. PLoS Negl Trop Dis 5:e1292. https://doi.org/10.1371/journal.pntd.0001292
Becker SL, Vogt J, Knopp S, Panning M, Warhurst DC, Polman K, Marti H, von Müller L, Yansouni CP, Jacobs J, Bottieau E, Sacko M, Rijal S, Meyanti F, Miles MA, Boelaert M, Lutumba P, van Lieshout L, N’Goran EK, Chappuis F, Utzinger J (2013) Persistent digestive disorders in the tropics: causative infectious pathogens and reference diagnostic tests. BMC Infect Dis 13:37. https://doi.org/10.1186/1471-2334-13-37
Becker SL, Piraisoody N, Kramme S, Marti H, Silué KD, Panning M, Nickel B, Kern WV, Herrmann M, Hatz CF, N’Goran EK, Utzinger J, von Müller L (2015) Real-time PCR for detection of Strongyloides stercoralis in human stool samples from Côte d'Ivoire: diagnostic accuracy, inter-laboratory comparison and patterns of hookworm co-infection. Acta Trop 150:210–217. https://doi.org/10.1016/j.actatropica.2015.07.019
Becker SL, Yap P, Horié NS, Alirol E, Barbé B, Bhatta NK, Bhattarai NR, Bottieau E, Chatigre JK, Coulibaly JT, Fofana HKM, Jacobs J, Karki P, Khanal B, Knopp S, Koirala K, Mahendradhata Y, Mertens P, Meyanti F, Murhandarwati EH, N’Goran EK, Peeling RW, Pradhan B, Ravinetto R, Rijal S, Sacko M, Saye R, Schneeberger PHH, Schurmans C, Silué KD, Steinmann P, van Loen H, Verdonck K, van Lieshout L, von Müller L, Yao JA, Boelaert M, Chappuis F, Polman K, Utzinger J (2016) Experiences and lessons from a multicountry NIDIAG study on persistent digestive disorders in the tropics. PLoS Negl Trop Dis 10:e0004818. https://doi.org/10.1371/journal.pntd.0004818
Belhassen-García M, Alonso-Sardón M, Martinez-Perez A, Soler C, Carranza-Rodriguez C, Pérez-Arellano JL, Muro A, Salvador F, Soil-Transmitted Helminths Study Group of the SEMTSI (2017) Surveillance of strongyloidiasis in Spanish in-patients (1998-2014). PLoS One 12:e0189449. https://doi.org/10.1371/journal.pone.0189449
Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Munoz J, Krolewiecki AJ, Gotuzzo E, Mena MA, Chiodini PL, Anselmi M Moreira J, Albonico M (2013) Strongyloides stercoralis: a plea for action. PLoS Negl Trop Dis 7:e2214. https://doi.org/10.1371/journal.pntd.0002214
Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, Albonico M, Gobbo M, Bonafini S, Angheben A, Requena-Mendez A, Muñoz J, Nutman TB (2014) Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 8:e2640. https://doi.org/10.1371/journal.pntd.0002640
Buonfrate D, Requena-Méndez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, Bisoffi Z (2013) Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis 13:78. https://doi.org/10.1186/1471-2334-13-78
Buonfrate D, Mena MA, Angheben A, Requena-Méndez A, Muñoz J, Gobbi F, Albonico M, Gotuzzo E, Bisoffi Z (2015) Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect 143:452–460. https://doi.org/10.1017/S0950268814001563
Buonfrate D, Baldissera M, Abrescia F, Bassetti M, Caramaschi G, Giobbia M, Mascarello M, Rodari P, Scattolo N, Napoletano G, Bisoffi Z, on behalf of the CCM Strongyloides Study Group (2016) Epidemiology of Strongyloides stercoralis in northern Italy: results of a multicentre case-control study, February 2013 to July 2014. Euro Surveill 21. https://doi.org/10.2807/1560-7917.ES.2016.21.31.30310
Buonfrate D, Perandin F, Formenti F, Bisoffi Z (2017) A retrospective study comparing agar plate culture, indirect immunofluorescence and real-time PCR for the diagnosis of Strongyloides stercoralis infection. Parasitology 144:812–816. https://doi.org/10.1017/S0031182016002559
Buonfrate D, Requena-Méndez A, Angheben A, Cinquini M, Cruciani M, Fittipaldo A, Giorli G, Gobbi F, Piubelli C, Bisoffi Z (2018) Accuracy of molecular biology techniques for the diagnosis of Strongyloides stercoralis infection – a systematic review and meta-analysis. PLoS Negl Trop Dis 12:e0006229. https://doi.org/10.1371/journal.pntd.0006229
Campo Polanco L, Gutiérrez LA, Cardona Arias J (2014) Diagnosis of Strongyloides stercoralis infection: meta-analysis on evaluation of conventional parasitological methods (1980-2013). Rev Esp Salud Publica 88:581–600 [in Spanish]. https://doi.org/10.4321/S1135-57272014000500004
Concha R, Harrington W Jr, Rogers AI (2005) Intestinal strongyloidiasis: recognition, management, and determinants of outcome. J Clin Gastroenterol 39:203–211
Depkes RI (Indonesian Ministry of Health) (2009) Pencapaian Kegiatan Pengendalian Penyakit dan Penyehatan Lingkungan di dalam Profil Pemberantasan Penyakit Menular dan Penyehatan lingkungan 2008, hal 19-173. Jakarta, Indonesia. [in Bahasa Indonesia]
Dunn JC, Turner HC, Tun A, Anderson RM (2016) Epidemiological surveys of, and research on, soil-transmitted helminths in Southeast Asia: a systematic review. Parasit Vectors 9:31. https://doi.org/10.1186/s13071-016-1310-2
Ghasemikhah R, Tabatabaiefar MA, Shariatzadeh SA, Shahbazi A, Hazratian T (2017) A PCR-based molecular detection of Strongyloides stercoralis in human stool samples from Tabriz City, Iran. Sci Pharm 85. https://doi.org/10.3390/scipharm85020017
Janwan P, Intapan PM, Thanchomnang T, Lulitanond V, Anamnart W, Maleewong W (2011) Rapid detection of Opisthorchis viverrini and Strongyloides stercoralis in human fecal samples using a duplex real-time PCR and melting curve analysis. Parasitol Res 109:1593–1601. https://doi.org/10.1007/s00436-011-2419-z
Jourdan PM, Lamberton PHL, Fenwick A, Addiss DG (2017) Soil-transmitted helminth infections. Lancet 391:252–265. https://doi.org/10.1016/S0140-6736(17)31930-X
Keiser PB, Nutman TB (2004) Strongyloides stercoralis in the immunocompromised population. Clin Microbiol Rev 17:208–217
Knopp S, Salim N, Schindler T, Karagiannis Voules DA, Rothen J, Lweno O, Mohammed AS, Singo R, Benninghoff M, Nsojo AA, Genton B, Daubenberger C (2014) Diagnostic accuracy of Kato-Katz, FLOTAC, Baermann, and PCR methods for the detection of light-intensity hookworm and Strongyloides stercoralis infections in Tanzania. Am J Trop Med Hyg 90:535–545. https://doi.org/10.4269/ajtmh.13-0268
Kramme S, Nissen N, Soblik H, Erttmann K, Tannich E, Fleischer B, Panning M, Brattig N (2011) Novel real-time PCR for the universal detection of Strongyloides species. J Med Microbiol 60:454–458. https://doi.org/10.1099/jmm.0.025338-0
Meurs L, Polderman AM, Vinkeles Melchers NV, Brienen EA, Verweij JJ, Groosjohan B, Mendes F, Mechendura M, Hepp DH, Langenberg MC, Edelenbosch R, Polman K, van Lieshout L (2017) Diagnosing polyparasitism in a high-prevalence setting in Beira, Mozambique: detection of intestinal parasites in fecal samples by microscopy and real-time PCR. PLoS Negl Trop Dis 11:e0005310. https://doi.org/10.1371/journal.pntd.0005310
Moghaddassani H, Mirhendi H, Hosseini M, Rokni M, Mowlavi G, Kia E (2011) Molecular diagnosis of Strongyloides stercoralis infection by PCR detection of specific DNA in human stool samples. Iran J Parasitol 6:23–30
Paula FM, Malta Fde M, Marques PD, Sitta RB, Pinho JR, Gryschek RC, Chieffi PP (2015) Molecular diagnosis of strongyloidiasis in tropical areas: a comparison of conventional and real-time polymerase chain reaction with parasitological methods. Mem Inst Oswaldo Cruz 110:272–274. https://doi.org/10.1590/0074-02760140371
Polman K, Becker SL, Alirol E, Bhatta NK, Bhattarai NR, Bottieau E, Bratschi MW, Burza S, Coulibaly JT, Doumbia MN, Horié NS, Jacobs J, Khanal B, Landouré A, Mahendradhata Y, Meheus F, Mertens P, Meyanti F, Murhandarwati EH, N’Goran EK, Peeling RW, Ravinetto R, Rijal S, Sacko M, Saye R, Schneeberger PHH, Schurmans C, Silué KD, Thobari JA, Traoré MS, van Lieshout L, van Loen H, Verdonck K, von Müller L, Yansouni CP, Yao JA, Yao PK, Yap P, Boelaert M, Chappuis F, Utzinger J (2015) Diagnosis of neglected tropical diseases among patients with persistent digestive disorders (diarrhoea and/or abdominal pain ≥ 14 days): a multi-country, prospective, non-experimental case-control study. BMC Infect Dis 15:338. https://doi.org/10.1186/s12879-015-1074-x
Repetto SA, Ruybal P, Batalla E, López C, Fridman V, Sierra M, Radisic M, Bravo PM, Risso MG, González Cappa SM, Alba Soto CD (2018) Strongyloidiasis outside endemic areas: long-term parasitological and clinical follow-up after ivermectin treatment. Clin Infect Dis 66:1558–1565. https://doi.org/10.1093/cid/cix1069
Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J (2013) The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis 7:e2002. https://doi.org/10.1371/journal.pntd.0002002
Salakory M, Soeyoko, Mardihusodo SJ, Sutanto (2013) Penggunaan teknologi remote sensing dan SIG untuk pengendalian dinamika populasi soil-transmitted helminths di satuan lahan endemis Pulau Ambon. J Manusia dan Lingkungan 20:339–352 [in Bahasa Indonesia]
Saugar JM, Merino FJ, Martin-Rabadan P, Fernandez-Soto P, Ortega S, Garate T, Rodriguez E (2015) Application of real-time PCR for the detection of Strongyloides spp. in clinical samples in a reference center in Spain. Acta Trop 142:20–25. https://doi.org/10.1016/j.actatropica.2014.10.020
Schär F, Odermatt P, Khieu V, Panning M, Duong S, Muth S, Marti H, Kramme S (2013a) Evaluation of real-time PCR for Strongyloides stercoralis and hookworm as diagnostic tool in asymptomatic schoolchildren in Cambodia. Acta Trop 126:89–92. https://doi.org/10.1016/j.actatropica.2012.12.012
Schär F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, Vounatsou P, Odermatt P (2013b) Strongyloides stercoralis: global distribution and risk factors. PLoS Negl Trop Dis 7:e2288. https://doi.org/10.1371/journal.pntd.0002288
Sharifdini M, Mirhendi H, Ashrafi K, Hosseini M, Mohebali M, Khodadadi H, Kia EB (2015) Comparison of nested polymerase chain reaction and real-time polymerase chain reaction with parasitological methods for detection of Strongyloides stercoralis in human fecal samples. Am J Trop Med Hyg 93:1285–1291. https://doi.org/10.4269/ajtmh.15-0309
Strkolcova G, Goldova M, Bockova E, Mojzisova J (2017) The roundworm Strongyloides stercoralis in children, dogs, and soil inside and outside a segregated settlement in Eastern Slovakia: frequent but hardly detectable parasite. Parasitol Res 116:891–900. https://doi.org/10.1007/s00436-016-5362-1
Utzinger J, Becker SL, Knopp S, Blum J, Neumayr AL, Keiser J, Hatz CF (2012) Neglected tropical diseases: diagnosis, clinical management, treatment and control. Swiss Med Wkly 142:w13727. https://doi.org/10.4414/smw.2012.13727
Vadlamudi RS, Chi DS, Krishnaswamy G (2006) Intestinal strongyloidiasis and hyperinfection syndrome. Clin Mol Allergy 4:8. https://doi.org/10.1186/1476-7961-4-8
Verweij JJ, Canales M, Polman K, Ziem J, Brienen EA, Polderman AM, van Lieshout L (2009) Molecular diagnosis of Strongyloides stercoralis in faecal samples using real-time PCR. Trans R Soc Trop Med Hyg 103:342–346. https://doi.org/10.1016/j.trstmh.2008.12.001
Widjana DP, Sutisna P (2000) Prevalence of soil-transmitted helminth infections in the rural population of Bali, Indonesia. Southeast Asian J Trop Med Public Health 31:454–459
Wongratanacheewin S, Pumidonming W, Sermswan RW, Pipitgool V, Maleewong W (2002) Detection of Opisthorchis viverrini in human stool specimens by PCR. J Clin Microbiol 40:3879–3880
Acknowledgements
We thank all participants in Maluku Tengah Regency, Maluku, Indonesia, and the staff from the Province Health Office of Maluku who facilitated the field work. We are grateful to the laboratory technicians and research staff involved in the field work both from RSUD Tulehu, Maluku Tengah Regency, Maluku, Indonesia and the Department of Parasitology at Universitas Gadjah Mada, Yogyakarta, for laboratory analyses.
Funding
This work is part of the NIDIAG European research network (Collaborative Project), supported by the European Union’s Seventh Framework Programme for research, technological development and demonstration under grant agreement no. 260260.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The protocol for the NIDIAG study on persistent digestive disorders was approved by the institutional review boards (IRBs) of the Institute of Tropical Medicine (ITM; Antwerp, Belgium) and Swiss Tropical and Public Health Institute (Swiss TPH; Basel, Switzerland) prior to external review. Approval in Indonesia was granted by the ethics committee of the Universitas Gadjah Mada (21 November 2013). The NIDIAG study is registered on ClinicalTrials.gov (identifier: NCT02105714). All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was obtained from all individual participants included in the study.
Additional information
Section Editor: Neil Bruce Chilton
Rights and permissions
About this article
Cite this article
Kristanti, H., Meyanti, F., Wijayanti, M.A. et al. Diagnostic comparison of Baermann funnel, Koga agar plate culture and polymerase chain reaction for detection of human Strongyloides stercoralis infection in Maluku, Indonesia. Parasitol Res 117, 3229–3235 (2018). https://doi.org/10.1007/s00436-018-6021-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-018-6021-5