Abstract
Ichthyophthirius is a severe disease of farmed freshwater fish caused by the parasitic ciliate Ichthyophthirius multifiliis (Ich). This disease can lead to considerable economic loss, but the protein profiles in different developmental stages of the parasite remain unknown. In the present study, proteins from trophonts and theronts of Ich were identified by isobaric tags for relative and absolute quantitation (iTRAQ). A total of 2300 proteins were identified in the two developmental stages, of which 1520 proteins were differentially expressed. Among them, 84 proteins were uniquely expressed in the theronts stage, while 656 proteins were expressed only in trophonts. The differentially expressed proteins were catalogued (assorted) to various functions of Ich life cycle, including biological process, cellular component, and molecular function that occur at distinct stages. Using a 1.5-fold change in expression as a physiologically significant benchmark, a lot of differentially expressed proteins were reliably quantified by iTRAQ analysis. Two hundred forty upregulated and 57 downregulated proteins in the trophonts stage were identified as compared with theronts. The identified proteins were involved in various functions of the I. multifiliis life cycle, including binding, catalytic activity, structural molecule activity, and transporter activity. Further investigation of the transcriptional levels of periplasmic immunogenic protein, transketolase, zinc finger, isocitrate dehydrogenase, etc., from the different protein profiles using quantitative RT-PCR showed identical results to the iTRAQ analysis. This work provides an effective resource to further our understanding of Ich biology, and lays the groundwork for the identification of potential drug targets and vaccines candidates for the control of this devastating fish pathogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ichthyophthirius multifiliis (Ich), the etiologic agent of “white spot” disease, is a highly pathogenic ciliate that infects virtually all species of freshwater fish. While Ichthyophthirius poses a major threat to commercial aquaculture worldwide, there are currently no effective vaccines for disease prevention, and acceptable strategies for the treatment of large-scale outbreaks are not yet available (Traxler et al. 1998; Matthews 2005).
The life cycle of I. multifiliis comprises several morphologically distinct stages, each fulfilling discrete function in the life history of I. multifiliis (MacLennan 1935; Nigrelli et al. 1976; Ewing and Kocan 1992). The trophont is the feeding stage which resides on the basal layers of the skin and gill epithelia of fish. Following a growth period, it leaves the fish as tomonts, which then encyst and subsequently undergo multiple divisions, and finally release numerous free-swimming infective theronts. The theront attaches to fish skin and penetrates into the epidermis where it settles as a trophont and completes the life cycle. Killing the infective theront or the detached trophont with various antiprotozoal drugs can stop the reproductive cycle and prevent spread of the disease to other fish (Tucker and Robinson 1990; Schäperclaus 1991; Wise et al. 2004).
Although the expression of stage-specific genes in the I. multifiliis has been detected with various transcriptome, cDNA cloning, and RNA sequencing approaches (Cassidy-Hanley et al. 2011; Coyne et al. 2011), I. multifiliis biology at each developmental stage, including developmental regulation, nutrition metabolism, and cell cycle, remains unclear. Clearly, a more thorough understanding of the proteins and metabolic pathways associated with the different stages of the parasite life cycle is critical to the development of effective control strategies.
Proteomic approaches are available to identify proteins involved in complex biological progress and allow for large-scale screening of potential vaccine candidate, drug target proteins from parasites. Isobaric tag for relative and absolute quantization (iTRAQ) is a common technique used in proteomics (Cha et al. 2012; Marancik et al. 2013; Lu et al. 2014). iTRAQ, a stable isotope method for protein measurement by using mass spectrometry (Ross et al. 2004), can be used for the comparison of four or eight different samples at the same time (Ross et al. 2004; Pierce et al. 2008), With these advantages, the iTRAQ method has now attracted more attentions which are applied in various studies (Jeswin et al. 2016).
To obtain a more complete picture of the I. multifiliis proteome, in this study, we performed differential proteomics to identify differentially expressed proteins in the infective theront and the detached trophont developmental stages of I. multifiliis, which provided valuable clues and insights to guide future studies on parasite development. This study laid the foundation for the discovery of therapeutic targets and the development of vaccine against I. multifiliis.
Materials and methods
Parasites
The sources of I. multifiliis, its propagation on grass carp, and collection of the cysts have been described by Yao et al. (2014). Several heavily infected grass carp (Ctenopharyngodon idella) obtained from the aquatic fry farm of Zhejiang Institute Freshwater Fisheries in China were placed into filtered aquarium water for 30–60 min. Mature trophonts were allowed to dislodge from the host by body movements of the fish while in close proximity. Isolated trophonts were sieved to remove containing debri, and then harvested by low speed centrifugation at 252 g for 5 min. Harvested trophonts were randomly divided into two batches, one was used to proteomic assay, and the other placed in a plastic beaker with double aerated distilled water and incubated for 24 h at 23°C. The next day, cultures were examined by microscopic examination (×100 magnifications) to insure that development was complete. Theronts were then sieved and collected by centrifugation at 1500×g for 3 min.
Protein preparation
Prepared theront and trophont samples were ground into powder in liquid nitrogen, then 300 μg of the powder protein was diluted in STD buffer (4% SDS, 150 mM Tris-HCl, 100 mM DTT, pH 8.0), heated at 100°C for 5 min, and then sonicated. After centrifugation, the supernatants were collected and protein content was measured by using a BCA protein assay reagent (Beyotime Institute of Biotechnology, China). Protein digestion was conducted according to the FASP procedure (Wisniewski 2009). Briefly, each sample was placed on an ultrafiltration filter (30 kDa cutoff, Sartorius, Germany) containing 200 μL of UA buffer (8 M urea, 150 mM Tris-HCl, pH 8.0) followed by centrifugation at 14,000×g for 30 min and an additional washing step with 200 μL of UA buffer; the process was stopped by adding 100 μL UA buffer (contain 50 mM iodoacetamide), then incubated for 30 min at room temperature in the dark, 14,000×g for 30 min after washing twice by 100 μL of UA buffer and dissolution buffer (Applied Biosystems, Foster City, CA, USA); the protein suspensions were then digested with 40 μL of trypsin (Promega, Madison, WI, USA) buffer (2 μg trypsin in 40 μL dissolution buffer) at 37°C for 16–18 h and then centrifuged at 14,000×g for 30 min. The resulted peptides were collected and the peptide concentration was analyzed at OD280.
iTRAQ labeling and high-pH reversed-phase (HpH) fractionation
iTRAQ labeling was performed according to the manufacturer’s instructions (Applied Biosystems). Briefly, the peptide mixtures were reconstituted with 30 μL of iTRAQ dissolution buffer. Each sample (100 μg) was labeled according to iTRAQ Reagent-8plex Multiplex Kit (AB SCIEX). The iTRAQ-labeled peptides were subjected to high-pH reversed-phase fractionation in 1100 Series HPLC Value System (Agilent) with the following chromatographic conditions: buffer A (10 mM Ammonium acetate, pH 10.0) and buffer B (10 mM ammonium acetate, 90% v/v ACN, pH 10.0) were eluted at a flow rate of 1 mL/min according to gradient elution procedure. The elution process was monitored by measuring absorbances at 214 nm, and fractions were collected every 1 min. The collected fractions (approximately 30) were finally combined into ten pools. Each fraction was concentrated via vacuum centrifugation and reconstituted in 40 μL of 0.1% v/v trifluoroacetic acid. All samples were stored at −80°C until LC-MS/MS analysis.
LC–ESI-MS/MS analysis
The iTRAQ-labeled samples were then subjected to Orbitrap Elite mass spectrometer (Thermo Fisher Scientific, San Jose, CA, USA). A total of 1 μg of each sample was loaded onto Thermo Scientific EASY column (two columns) using an autosampler at a flow rate of 150 nL/min. The mass spectrometer was operated in positive ion mode, and MS spectra were acquired over a range of 300–2000 m/z. The resolving powers of the MS scan and MS/MS scan at 200 m/z for the Orbitrap Elite were set as 60,000 and 15,000, respectively. The top ten most intense signals in the acquired MS spectra were selected for further MS/MS analysis. The isolation window was 1 m/z, and ions were fragmented through higher energy collisional dissociation with normalized collision energies of 35 eV. The maximum ion injection times were set at 50 ms for the survey scan and 150 ms for the MS/MS scans, and the automatic gain control target values for full scan modes was set to 1.0 × 10−6 and for MS/MS was 5 × 104. The dynamic exclusion duration was 30 s.
Protein data analysis
The raw MS data files were converted using Proteome Discoverer 1.4 software (Thermo Fisher Scientific). Protein identification was performed using the Mascot software Matrix 2.3.02 (Science, London, UK) against the I. multifiliis database downloading from UniProt of EMBL. The differentially expressed proteins were defined as those with 1.5-fold change relative to one another, with p < 0.05. The gene name, functional annotations, protein orthologous classification, molecular interaction, and reaction networks were analyzed by Blast, Gene Ontology, and KEGG Pathway, respectively.
Quantitative real-time RT-PCR verification
Total RNA was extracted from theronts and trophonts by TRIzol Reagent (Simgen). cDNA was then synthesized using the Reverse Transcriptase M-MLV Kit (TaKaRa) following the instructions. The real-time quantitative PCR was performed using THUNDERBIRD SYBR qPCR Mix Kit (Toyobo), and carried out in astratagene MxProSystem (Stratagene mx3005p, USA) in 96-well reaction plates. All PCRs were performed at least three times. Additional dissociation-curve analysis was performed and showed a single melting curve in all cases. Data were analyzed by the Stratagene MxPro software (Stratagene mx3005p, USA).
Results
Identification of differentially expressed proteins
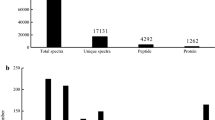
The spectra generated from the iTRAQ experiment matched 2300 proteins through BLAST in GenBank and NCBI. The differentially expressed proteins (DEPs) in the two developmental stages (theronts and trophonts) were analyzed using comparison of their identified spectra. A total of 1520 proteins were differentially expressed in the two arbitrary stages. Gene ontology (GO) analysis of total proteins was based on cellular component, biological process, and molecular function. Differentially expressed proteins were also identified using GO for gene function classification. The molecular function ontology analysis was as follows: binding (trophonts 45.76%; theronts 42.69%,), catalytic activity (39.09%; 38.95%), structural molecule activity (5.99%; 7.36%), transporter activity (4.36%; 5.66%), electron carrier activity (1.25%; 1.60%), and molecular function regulator (1.81%; 1.71%). The percentages of proteins classified into bonding and catalytic activities were the highest among molecular functions, which were up to 84.85 and 81.64% for theronts and trophonts, respectively. The GO analysis of the identified proteins was shown in Fig. 1.
Clusters of Orthologous Groups of proteins (COG) classification in the two developmental stages revealed that the proteins were primarily involved in signal transduction mechanisms, translation, ribosomal structure and biogenesis, posttranslational modification, protein turnover, chaperones, general function prediction, energy production and conversion, lipid transport and metabolism, amino acid transport and metabolism, carbohydrate transport and metabolism, nucleotide transport and metabolism, and replication, recombination, and repair (Fig. 2). The results indicated that proteins from the two developmental stages were involved in every aspect of parasite growth and metabolism.
Protein profiling of the stage-specific differentially expressed proteins
Among the differentially expressed proteins, 84 proteins were unique expressed in theronts stage (Table 1), including Ymf75 (mitochondrion, accession no. 345894133), flagellar microtugule protofilament ribbon protein (accession no. 471219372), intraflagellar transport protein (accession no. 471221951), oxoglutarate dehydrogenase (accession no. 471228421), radial spoke head protein, 471232003, kinesin family member 9 (accession no. 471234104), phosphoglycerate kinase (471221776), and inorganic pyrophosphatase (accession no. 471223495) papain family cysteine protease (accession no. 471233695). As for trophonts (Table 2), there were 656 specific proteins only expressed in this development such as carbon-nitrogen family protein (accession no. 471226451), tryptophanyl-tRNA synthetase (accession no .471226600), Ran binding protein (accession no. 471226744) DNA-directed polymerase II polypeptide J (accession no. 471227247), zinc-binding dehydrogenase family protein (accession no. 471228852), copper/zinc superoxide dismutase family protein (accession no. 471230086), and parkin co-regulated gene protein (accession no. 471235274). Stage-specific differentially expressed proteins were also identified using GO for gene function classification, and the cellular component ontology analysis was listed in Figs. 3 and 4.
iTRAQ quantification
Using a 1.5-fold change in expression as a physiologically significant benchmark, a lot of differentially expressed proteins were reliably quantified by iTRAQ analysis. Two hundred forty upregulated and 57 downregulated proteins in the trophonts stage were identified as compared with theronts. The proteins expressed at a high level in trophonts were mainly propionates—ligase, kap beta 3 protein, ribosomal protein, zinc-binding dehydrogenase family protein, phenylalanyl-tRNA alpha subunit (Table 1). The proteins that were more abundant in theront than trophonts were K antiporter P-type alpha subunit family protein, ornithine aminotransferase, IQ calmodulin-binding motif family protein, normocyte-binding protein, etc. (Table 2).
Quantitative real-time RT-PCR analysis validation of DEPs
Six genes from DEPs designated redoxin domain protein (471224523), protein kinase domain protein (471227705), spfh domain band 7 family protein (471226098), normocyte-binding protein (471219697), immobilization antigen isoform (471231290), and leishmanolysin family protein (471230932) were selected for RT-PCR analysis to quantify their transcriptional levels (Fig. 5). The results revealed that the genes encoding normocyte-binding protein, protein kinase domain protein, and spfh domain band 7 family protein were significantly higher expressed in theronts than in trophonts stages. The redoxin domain protein was upregulated in trophonts than in theronts. Leishmanolysin family protein (471230932) only expressed in trophonts, but in contrast to iTRAQ analysis, immobilization antigen isoform (471231290) were expressed in both development stages. The PCR results were mainly consistent with the iTRAQ analysis, which suggested that the proteomics analysis tool used in this study is reliable.
Confirmation of differentially expressed genes of Ichthyophthirius multifiliis by RT-PCR. Note: RDP24523: redoxin domain protein, PDP27705: protein kinase domain protein, SBS26098: spfh domain band 7 family protein, NBP19697: normocyte-binding protein, IAI31290: immobilization antigen isoform, LFP30932: leishmanolysin family protein
Discussions
In recent years, an increasing number of ichthyophthiriosis outbreaks have led to substantial economic loss in fisheries and aquaculture (Yao et al. 2010, 2011). Numerous strategies including immunoprophylaxis and chemotherapeutics had been used to control the parasite; however, no methods resulted in great success. Therefore, it is necessary to screen new drug targets and vaccine candidates of I. multifiliis, and the understanding of the life-stage biology, host pathogen interactions, signal transduction, and regulation, the key enzyme invasion, and metabolic pathway of the parasite can facilitate further studies. Numerous proteins are involved in differential regulation of distinct life cycle stages of I. multifiliis. The differentially expressed genes at each developmental stage have been identified in previous studies (Abernathy et al. 2011). However, the protein profiles in their developmental stages remain largely unknown. Herein, we applied a quantitative proteomics approach with the iTRAQ technique to fully reveal the protein expression profiles of the two developmental stages (theronts and trophonts). A total of 1520 proteins were identified involved in binding, catalytic activity, structural molecule activity, transporter activity, electron carrier activity, and molecular function regulator.
Among the differentially expressed proteins in the two development stages of I. multifiliis, 84 of them were uniquely expressed in the theronts stage. In line with the previous study, flagellar microtugule protofilament ribbon protein, intraflagellar transport protein, kinesin family member 9 were highly expressed in the theronts stage (Table 2), which almost certainly reflects the critical energy needs of these cells for rapid and sustained motility associated with host finding (Hennessey et al. 2002; Wood et al. 2007; Cassidy-Hanley et al. 2011). Another specific expressed protein in theronts, phosphoglycerate kinase (PGK), has traditionally been studied as the sixth enzyme of the glycolytic pathway in which it equilibrates phosphate transfer between position 1 of 1,3-bisphosphoglycerate and the g-phosphate of MgATP2−. PGK has also been shown to influence DNA replication and repair in mammalian cell nuclei (Vishwanatha et al. 1992; Popanda et al. 1998) and stimulate viral messenger RNA synthesis in the cytosol (Ogino et al. 1999). Interestingly, the leishmanolysin family protein (accession no. 471230932) that contribute to invasion, migration, and adhesion of Leishmania species within the extracellular matrix of host tissues (McGwire et al. 2003; d’Avila-Levy et al. 2008) were also highly expressed in the theronts stage. As for trophonts, we found 656 specific expressed proteins mainly associated with the cellular process and binding and catalytic activity. ATP synthase alpha subunit precursor (47122655), the purine salvage, and metabolism enzymes were highly expressed only in the trophonts stage; as we know, enzymes involved in purine salvage are of particular interest since a variety of parasitic protozoa are incapable of synthesizing purines de novo, so it may be an attractive target for pharmacological intervention in parasite control (Abernathy et al. 2007; Sullivan et al. 2005). These unique proteins may offer additional targets for vaccine and therapeutic drug development.
Proteases in parasitic protozoa have long been considered potential drug targets due to their crucial roles in parasite development and infection, and the feasibility of designing specific inhibitors (Atkinson et al. 2009; Blackman 2008; Kuang et al. 2009; Wu et al. 2003). Onara et al. (2008) reported that carp infected with Ich could increase the expression levels of a2M3, a protease inhibitor of endogenous and exogenous proteases which indicated that anti-proteases could be viable anti-infectives. In the present study, we identified 105 protease homologs from the two development stages of Ich. The inorganic pyrophosphatase, oxoglutarate dehydrogenase, phosphoglycerate kinase, isocitrate dehydrogenase, and transketolase and lactate dehydrogenase were significantly downregulated in the theront as compared to the trophont stage, which indicated maximal activation of gluconeogenesis in theront which reflects the more energy needs of the parasite during invasion and migration (Janke and Kneussel 2010). Though our study identified only half of the protease homologs revealed by comparative genomic analysis (Coyne et al. 2011), it significantly expands the range of protease targets. More functions of the proteins should be further investigated.
For proteins of known function, their differential expression often reflects the biological activities associated with each stage of the parasite life cycle. In the present study, the normocyte-binding protein (71219697) was about 10 times more prevalent in theronts than in trophonts. Normocyte-binding proteins are members of reticulocyte-binding proteins which are characterized in malaria parasite species including Plasmodium cynomolgi (Okenu 2005) and Plasmodium yoelii (Ogun 2011). It was identified as a key mediator of Plasmodium knowlesi human infection and may be a target for vaccine development against this emerging pathogen (Ahmed et al. 2016). Karyopherin (kap) beta 3 protein (471220389), an import receptor for cargo interacts in the cytoplasm and nuclear, is transported through the nuclear pore complex, and is released inside the nucleus (Moroianu 1999; Nakielny and Dreyfuss 1999; Wente 2000). Coyne et al. (2008) reported that there are 88% as many mitochondrial carriers in Ich as in Tetrahymena. They mention that such a high representation of these carriers suggests a strong dependence of Ich’s energy generation on mitochondrial aerobic respiration. It may become an attractive drug target because Ich mitochondrial ATP synthase is highly divergent from its vertebrate form. As mentioned in Table 1, calmodulin-binding motif family protein (471235683) was highly prevalent in theronts than in trophont. As described by Coyne et al. (2008), Ich and other ciliates contain a large number of calcium- and calmodulin-regulated protein kinases, which is absent in vertebrates. Calcium-regulated pathways have come under study as promising therapeutic targets against apicomplexan parasites (Nagamune et al. 2008; Billker et al. 2009) and also could be considered as targets against Ich.
In conclusion, a total of 2300 proteins were identified by iTRAQ technique in the two developmental stages, and 1520 proteins were differentially expressed in the trophonts stage compared with theronts. GO analysis revealed that some proteins are involved in important functions related to biological process, cellular component, and molecular function that occur at distinct stages. This work provides an effective resource to further our understanding of Ich biology, and lays the groundwork for the identification of potential drug targets and vaccines candidates for the control of this devastating fish pathogen.
References
Abernathy JW, Xu P, Li P (2007) Generation and analysis of expressed sequence tags from the ciliate protozoan parasite Ichthyophthirius multifiliis. BMC Genomics 8:176
Abernathy J, Xu DH, Peatman E, Kucuktas H, Klesius P, Liu ZJ (2011) Gene expression profiling of a fish parasite Ichthyophthirius multifiliis: insights into development and senescence-associated avirulence. Comp Biochem Phy D 6:382–392
Ahmed M, Fong A, Yee M, Lau YL, Yusof R (2016) Clustering and genetic differentiation of the normocyte binding protein (nbpxa) of Plasmodium knowlesi clinical isolates from Peninsular Malaysia and Malaysia Borneo. Malar J 15:241
Atkinson HJ, Babbitt PC, Sajid M (2009) The global cysteine peptidase landscape in parasites. Trends Parasitol 25:573–581
Billker O, Lourido S, Sibley LD (2009) Calcium-dependent signaling and kinases in apicomplexan parasites. Cell Host Microbe 5:612–622
Blackman MJ (2008) Malarial proteases and host cell egress: an ‘emerging’ cascade. Cell Microbiol 10:1925–1934
Cassidy-Hanley DM, Pratt CM, Pratt LH, Devine C, Hossain MM, Dickerson HW, Clark TG (2011) Transcriptional profiling of stage specific gene expression in the parasitic ciliate Ichthyophthirius multifiliis. Mol Biochem Parasit 178:29–39
Cha IS, Kwon J, Park SH (2012) Kidney proteome responses in the teleost fish Paralichthys olivaceus indicate a putative immune response against Streptococcus parauberis, J. Proteome 75:5166–5175
Coyne RS, Thiagarajan M, Jones KM, Wortman JR, Tallon LJ, Haas BJ, Cassidy-Hanley DM, Wiley EA, Smith JJ, Collins K, Lee SR, Couvillion MT, Liu Y, Garg J (2008) Refined annotation and assembly of the Tetrahymena thermophila genome sequence through EST analysis, comparative genomic hybridization, and targeted gap closure. BMC Genomics 9:562
Coyne RS, Hannick L, Shanmugam D, Hostetler JB, Brami D, Joardar VS, Johnson J, Radune D, Singh I, Badger JH, Kumar U, Saier M, Wang YF, Cai H, Gu JG, Mather MW, Vaidya AB, Wilkes DE, Rajagopalan V, Asai DJ, Pearson CG, Findly RC, Dickerson HW, Wu M, Martens C, de Peer YV, Roos DS, Cassidy-Hanley DM, Clark TG (2011) Comparative genomics of the pathogenic ciliate Ichthyophthirius multifiliis, its free-living relatives and a host species provide insights into adoption of a parasitic lifestyle and prospects for disease control. Genome Biol 12:1–26
d’Avila-Levy CM, Santos LO, Marinho FA (2008) Crithidia deanei: influence of parasite gp63 homologue on the interaction of endosymbiont-harboring and aposymbiotic strains with Aedes aegypti midgut. Exp Parasitol 118:345–353
Ewing MS, Kocan KM (1992) Invasion and development strategies of Ichthyophthirius multifiliis, a parasitic ciliate of fish. Parasitol Today 8:204–208
Hennessey TM, Kim DY, Oberski DJ, Hard R, Rankin SA, Pennock DG (2002) Inner arm dynein 1 is essential for Ca++-dependent ciliary reversals in Tetrahymena thermophila. Cell Motil Cytoskel 53:281–288
Janke C, Kneussel M (2010) Tubulin post-translational modifications: encoding functions on the neuronal microtubule cytoskeleton. Trends Neurosci 33:362–372
Jeswin J, Xie XL, Ji QL, Wang KJ, Liu HP (2016) Proteomic analysis by iTRAQ in red claw crayfish, Cherax quadricarinatus, hematopoietic tissue cells post white spot syndrome virus infection. Fish Shellfish Immunol 50:288–296
Kuang R, Gu J, Cai H, Wang Y (2009) Improved prediction of malaria degradomes by supervised learning with SVM and profile kernel. Genetica 136:189–209
Lu AJ, Hu XC, Wang Y (2014) iTRAQ analysis of gill proteins from the zebrafish (Danio rerio) infected with Aeromonas hydrophila. Fish Shellfish Immunol 36:229–239
MacLennan RF (1935) Observations on the life cycle of Ichthyophthirius, a ciliate parasitic on fish. Northwestern Scientist 9:12–14
Marancik DP, Fast MD, Camus AC (2013) Proteomic characterization of the acute- phase response of yellow stingrays Urobatis jamaicensis after injection with a Vibrio anguillarum-ordalii bacterin. Fish Shellfish Immunol 34:1383–1389
Matthews RA (2005) Ichthyophthirius multifiliis Fouquet and ichthyophthiriosis in freshwater teleosts. Adv Parasitol 59:159–241
McGwire BS, Chang KP, Engman DM (2003) Migration through the extracellular matrix by the parasitic protozoan Leishmania is enhanced by surface metallo protease gp 63. Infect Immun 71:1008–1010
Moroianu J (1999) Nuclear import and export pathways. J Cell Biochem 76–83
Nagamune K, Moreno SN, Chini EN, Sibley LD (2008) Calcium regulation and signaling in apicomplexan parasites. Subcell Biochem 47:70–81
Nakielny S, Dreyfuss G (1999) Transport of proteins and RNAs in and out of the nucleus. Cell 99:677–690.
Nigrelli RF, Pokorny KS, Ruggieri GD (1976) Notes on Ichthyophthirius multifiliis, a ciliate parasitic on freshwater fishes, with some remarks on possible physiological races and species. Trans Am Microsc Soc 95:607–613
Ogino T, Iwama M, Kinouchi J (1999) Involvement of a cellular glycolytic enzyme, phosphoglycerate kinase, in Sendai virus transcription. J Biol Chem 274:35999–36008
Ogun SA (2011) Targeted disruption of py235ebp-1: invasion of erythrocytes by Plasmodium yoelii using an alternative Py235 erythrocyte binding protein. PLoS Pathog 7(2):1001288
Okenu DM (2005) The reticulocyte binding proteins of Plasmodium cynomolgi: a model system for studies of P. vivax. Mol Biochem Parasitol 143(1):116–120
Onara DF, Forlenza M, Gonzalez SF, Rakus KL, Pilarczyk A, Irnazarow I, Wiegertjes GF (2008) Differential transcription of multiple forms of alpha-2- macroglobulin in carp (Cyprinus carpio) infected with parasites. Dev Comp Immunol 32:339–347
Pierce A, Unwin RD, Evans CA (2008) Eight-channel iTRAQ enables comparison of the activity of six leukemogenic tyrosine kinases. Mol Cell Proteomics 7:853–863
Popanda O, Fox G, Thielmann HW (1998) Modulation of DNA polymerases a, d and e by lactate dehydrogenase and 3-phosphoglycerate kinase. Biochim Biophys Acta 1397:102–117
Ross PL, Huang YLN, Marchese JN (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3:1154–1169
Schäperclaus W (1991) Diseases caused by ciliates. In: Schäperclaus W, Kulow H, Schreckenbach K (eds) Fish diseases. Published for the U.S. Department of the Interior and the National Science Foundation, Washington, DC. Amerind, New Delhi, pp 702–725
Sullivan JWJ, Dixon SE, Li C, Striepen B, Queener SF (2005) IMP dehydrogenase from the protozoan parasite Toxoplasma gondii. Antimicrob Agents Chemother 49:2172–2179
Traxler GS, Richard J, McDonald TE (1998) Ichthyophthirius multifiliis (Ich) epizootics in spawning sockeye salmon in British Columbia, Canada. J Aqua Ani Health 10:143–151
Tucker CS, Robinson EH (1990) Channel catfish farming handbook. Van Nostrand Reinhold, New York
Vishwanatha JK, Jindal HK, Davis RG (1992) The role of primer recognition proteins in DNA replication: association with nuclear matrix in HeLa cells. J Cell Sci 101:25–34
Wente SR (2000) Gatekeepers of the nucleus. Science 288:1374–1377
Wise DJ, Camus AC, Schwedler TE, Terhune JS (2004) Infectious diseases. In: Tucker CS, Hargreaves JA (eds) Biology and culture of channel catfish. Amsterdam 444:502
Wisniewski JR (2009) Universal sample preparation method for proteome analysis. Nat Methods 6(5):359–362
Wood CR, Hard R, Hennessey TM (2007) Targeted gene disruption of dynein heavy chain 7 of Tetrahymena thermophila results in altered ciliary waveform and reduced swim speed. J Cell Sci 120:3075–3085
Wu Y, Wang X, Liu X, Wang Y (2003) Data-mining approaches reveal hidden families of proteases in the genome of malaria parasite. Genome Res 13:601–616
Yao JY, Shen JY, Li XL, Xu Y, Hao GJ, Pan XY, Wang GX, Yin WL (2010) Effect of sanguinarine from the leaves of Macleaya cordata against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idella). Parasitol Res 107:1035–1042
Yao JY, Zhou ZM, Li XL, Yin WL, Ru HS, Pan XY, Hao GJ, Xu Y, Shen JY (2011) Antiparasitic efficacy of dihydrosanguinarine and dihydrochelerythrine from Macleaya microcarpa against Ichthyophthirius multifiliis in richadsin (Squaliobarbus curriculus). Vet Parasitol 183:8–13
Yao JY, Li XC, Li G, Xu Y, Ai WM, Shen JY (2014) Anti-parasitic activities of specific bacterial extracellular products of Streptomyces griseus SDX-4 against Ichthyophthirius multifiliis. Parasitol Res 113(8):3111–3117
Acknowledgments
The research was supported by the National Natural Science Foundation of China (31302211), the Commonness and Commonweal Technology Application project program of Zhejiang province (2014C32055).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yao, JY., Xu, Y., Yuan, XM. et al. Proteomic analysis of differentially expressed proteins in the two developmental stages of Ichthyophthirius multifiliis . Parasitol Res 116, 637–646 (2017). https://doi.org/10.1007/s00436-016-5328-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-016-5328-3