Abstract
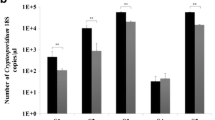
To compare phase contrast microscopy (PCM) of unstained slides for the detection of Cryptosporidium spp. oocysts with a commercially available enzyme immunoassay (EIA) for the detection of cryptosporidial antigen in human stool samples, we prospectively analysed by both methods 463 fresh human stool samples obtained from diarrhoeic patients between July and October 2014. Compared with the EIA, the sensitivity, specificity, positive and negative predictive value of PCM were 88.9 % (95 % confidence interval (CI), 66.0–98.1 %), 100 % (95 % CI, 99.0–100 %), 100 % (95 % CI, 77.3–100 %) and 99.6 % (95 % CI, 98.3–100 %), respectively. Additionally, we retrospectively examined with PCM 65 fixed stool samples that had been collected in 2010 from mostly asymptomatic Rwandan children <5 years of age; 14 of these samples had previously yielded positive results with a highly sensitive real-time (RT)-PCR. PCM detected cryptosporidia in 5/14 RT-PCR-positive samples, and notably, also in one of 51 RT-PCR-negative samples, which was subsequently confirmed by acid-fast staining. Positive and negative percent agreement of PCM with RT-PCR were 35.7 % (95 % CI, 16.2–61.4 %) and 98.0 % (95 % CI, 88.7–100 %), respectively. Positive PCM results were associated with higher RT-PCR cycle threshold values (p = 0.044). In conclusion, PCM offers a highly specific, undemanding and inexpensive method for the laboratory diagnosis of acute human cryptosporidiosis independent of the causative Cryptosporidium species.


Similar content being viewed by others
References
Abu Samra N, Thompson PN, Jori F, Frean J, Poonsamy B, du Plessis D, Mogoye B, Xiao L (2013) Genetic characterization of Cryptosporidium spp. in diarrhoeic children from four provinces in South Africa. Zoonoses Public Health 60(2):154–159
Adamu H, Petros B, Zhang G, Kassa H, Amer S, Ye J, Feng Y, Xiao L (2014) Distribution and clinical manifestations of Cryptosporidium species and subtypes in HIV/AIDS patients in Ethiopia. PLoS Negl Trop Dis 8(4):e2831
Baldursson S, Karanis P (2011) Waterborne transmission of protozoan parasites: review of worldwide outbreaks—an update 2004–2010. Water Res 45(29):6603–6614
Baxby D, Hart CA, Blundell N (1985) Shedding of oocysts by immunocompetent individuals with cryptosporidiosis. J Hyg (Lond) 95(3):703–709
Blanco MA, Iborra A, Vargas A, Nsie E, Mbá L, Fuentes I (2009) Molecular characterization of Cryptosporidium isolates from humans in Equatorial Guinea. Trans R Soc Trop Med Hyg 103(12):1282–1284
Bukhari Z, Smith HV (1997) Cryptosporidium parvum: oocyst excretion and viability patterns in experimentally infected lambs. Epidemiol Infect 119(1):105–108
Chalmers RM, Campbell BM, Crouch N, Charlett A, Davies AP (2011) Comparison of diagnostic sensitivity and specificity of seven Cryptosporidium assays used in the UK. J Med Microbiol 60(Pt 11):1598–1604
Checkley W, White AC Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER (2015) A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15(1):85–94
Eibach D, Krumkamp R, Al-Emran HM, Sarpong N, Hagen RM, Adu-Sarkodie Y, Tannich E, May J (2015) Molecular characterization of Cryptosporidium spp. among children in rural Ghana. PLoS Negl Trop Dis 9(3):e0003551
European Centre for Disease Prevention and Control (2014) Annual epidemiological report 2014—food- and waterborne diseases and zoonoses. ECDC, Stockholm
Fathy MM, Abdelrazek NM, Hassan FA, El-Badry AA (2014) Molecular copro-prevalence of Cryptosporidium in Egyptian children and evaluation of three diagnostic methods. Indian Pediatr 51(9):727–729
Fontaine M, Guillot E (2002) Development of a TaqMan quantitative PCR assay specific for Cryptosporidium parvum. FEMS Microbiol Lett 214(1):13–17
Gatei W, Wamae CN, Mbae C, Waruru A, Mulinge E, Waithera T, Gatika SM, Kamwati SK, Revathi G, Hart CA (2006) Cryptosporidiosis: prevalence, genotype analysis, and symptoms associated with infections in children in Kenya. Am J Trop Med Hyg 75(1):78–82
Heine J (1982) A simple technic for the demonstration of cryptosporidia in feces. Zentralbl Veterinarmed B 29(4):324–327
Helmy YA, Krücken J, Nöckler K, von Samson-Himmelstjerna G, Zessin KH (2014) Comparison between two commercially available serological tests and polymerase chain reaction in the diagnosis of Cryptosporidium in animals and diarrhoeic children. Parasitol Res 113(1):211–216
Ignatius R, Eisenblatter M, Regnath T, Mansmann U, Futh U, Hahn H, Wagner J (1997) Efficacy of different methods for detection of low Cryptosporidium parvum oocyst numbers or antigen concentrations in stool specimens. Eur J Clin Microbiol Infect Dis 16(10):732–736
Ignatius R, Gahutu JB, Klotz C, Steininger C, Shyirambere C, Lyng M, Musemakweri A, Aebischer T, Martus P, Harms G, Mockenhaupt FP (2012) High prevalence of Giardia duodenalis assemblage B infection and association with underweight in Rwandan children. PLoS Negl Trop Dis 6(6):e1677
Johnston SP, Ballard MM, Beach MJ, Causer L, Wilkins PP (2003) Evaluation of three commercial assays for detection of Giardia and Cryptosporidium organisms in fecal specimens. J Clin Microbiol 41(2):623–626
Kabayiza JC, Andersson ME, Nilsson S, Baribwira C, Muhirwa G, Bergstrom T, Lindh M (2014) Diarrhoeagenic microbes by real-time PCR in Rwandan children under 5 years of age with acute gastroenteritis. Clin Microbiol Infect 20(12):O1128–O1135
Kaushik K, Khurana S, Wanchu A, Malla N (2008) Evaluation of staining techniques, antigen detection and nested PCR for the diagnosis of cryptosporidiosis in HIV seropositive and seronegative patients. Acta Trop 107(1):1–7
Kehl KS, Cicirello H, Havens PL (1995) Comparison of four different methods for detection of Cryptosporidium species. J Clin Microbiol 33(2):416–418
Khurana S, Sharma P, Sharma A, Malla N (2012) Evaluation of Ziehl-Neelsen staining, auramine phenol staining, antigen detection enzyme linked immunosorbent assay and polymerase chain reaction, for the diagnosis of intestinal cryptosporidiosis. Trop Parasitol 2(1):20–23
Kimmig P, Hartmann S (1986) Ein einfacher Kryptosporidiennachweis im Rahmen der parasitologischen Stuhl-Routinediagnostik. Lab Med 10:285–286
Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, Wu Y, Sow SO, Sur D, Breiman RF, Faruque AS, Zaidi AK, Saha D, Alonso PL, Tamboura B, Sanogo D, Onwuchekwa U, Manna B, Ramamurthy T, Kanungo S, Ochieng JB, Omore R, Oundo JO, Hossain A, Das SK, Ahmed S, Qureshi S, Quadri F, Adegbola RA, Antonio M, Hossain MJ, Akinsola A, Mandomando I, Nhampossa T, Acacio S, Biswas K, O'Reilly CE, Mintz ED, Berkeley LY, Muhsen K, Sommerfelt H, Robins-Browne RM, Levine MM (2013) Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case–control study. Lancet 382(9888):209–222
Lobo ML, Augusto J, Antunes F, Ceita J, Xiao L, Codices V, Matos O (2014) Cryptosporidium spp., Giardia duodenalis, Enterocytozoon bieneusi and other intestinal parasites in young children in Lobata province, democratic republic of São Tomé and Principe. PLoS One 9(5):e97708
Martin-Ampudia M, Mariscal A, Lopez-Gigosos RM, Mora L, Fernandez-Crehuet J (2012) Under-notification of cryptosporidiosis by routine clinical and laboratory practices among non-hospitalised children with acute diarrhoea in Southern Spain. Infection 40(2):113–119
Molloy SF, Smith HV, Kirwan P, Nichols RA, Asaolu SO, Connelly L, Holland CV (2010) Identification of a high diversity of Cryptosporidium species genotypes and subtypes in a pediatric population in Nigeria. Am J Trop Med Hyg 82(4):608–613
Mor SM, Tzipori S (2008) Cryptosporidiosis in children in Sub-Saharan Africa: a lingering challenge. Clin Infect Dis 47(7):915–921
Morgan UM, Pallant L, Dwyer BW, Forbes DA, Rich G, Thompson RC (1998) Comparison of PCR and microscopy for detection of Cryptosporidium parvum in human fecal specimens: clinical trial. J Clin Microbiol 36(4):995–998
Morse TD, Nichols RA, Grimason AM, Campbell BM, Tembo KC, Smith HV (2007) Incidence of cryptosporidiosis species in paediatric patients in Malawi. Epidemiol Infect 135(8):1307–1315
Parisi MT, Tierno PM Jr (1995) Evaluation of new rapid commercial enzyme immunoassay for detection of Cryptosporidium oocysts in untreated stool specimens. J Clin Microbiol 33(7):1963–1965
Sagebiel D, Weitzel T, Stark K, Leitmeyer K (2009) Giardiasis in kindergartens: prevalence study in Berlin, Germany, 2006. Parasitol Res 105(3):681–687
Savioli L, Smith H, Thompson A (2006) Giardia and Cryptosporidium join the ‘neglected diseases initiative’. Trends Parasitol 22(5):203–208
Schuster W, Fischer R, Alsleben S, Schuster B (1991) Cryptosporidium sp. in stool specimens from diarrhoeic and asymptomatic individuals in the Magdeburg area (East Germany). Angew Parasitol 32(4):193–197
Shirley DA, Moonah SN, Kotloff KL (2012) Burden of disease from cryptosporidiosis. Curr Op Infect Dis 25(5):555–563
Tumwine JK, Kekitiinwa A, Nabukeera N, Akiyoshi DE, Rich SM, Widmer G, Feng X, Tzipori S (2003) Cryptosporidium parvum in children with diarrhea in Mulago Hospital, Kampala, Uganda. Am J Trop Med Hyg 68(6):710–715
Valentiner-Branth P, Steinsland H, Fischer TK, Perch M, Scheutz F, Dias F, Aaby P, Molbak K, Sommerfelt H (2003) Cohort study of Guinean children: incidence, pathogenicity, conferred protection, and attributable risk for enteropathogens during the first 2 years of life. J Clin Microbiol 41(9):4238–4245
Verweij JJ, Blange RA, Templeton K, Schinkel J, Brienen EA, van Rooyen MA, van Lieshout L, Polderman AM (2004) Simultaneous detection of Entamoeba histolytica, Giardia lamblia, and Cryptosporidium parvum in fecal samples by using multiplex real-time PCR. J Clin Microbiol 42(3):1220–1223
Wanyiri JW, Kanyi H, Maina S, Wang DE, Steen A, Ngugi P, Kamau T, Waithera T, O'Connor R, Gachuhi K, Wamae CN, Mwamburi M, Ward HD (2014) Cryptosporidiosis in HIV/AIDS patients in Kenya: clinical features, epidemiology, molecular characterization and antibody responses. Am J Trop Med Hyg 91(2):319–328
Weber R, Bryan RT, Bishop HS, Wahlquist SP, Sullivan JJ, Juranek DD (1991) Threshold of detection of Cryptosporidium oocysts in human stool specimens: evidence for low sensitivity of current diagnostic methods. J Clin Microbiol 29(7):1323–1327
Yoder JS, Beach MJ (2010) Cryptosporidium surveillance and risk factors in the United States. Exp Parasitol 124(1):31–39
Zaidah AR et al (2008) Detection of Cryptosporidium parvum in HIV-infected patients in Malaysia using a molecular approach. Southeast Asian J Trop Med Public Health 39(3):511–516
Acknowledgements
This study was in part supported by the German Federal Ministry for Economic Cooperation and Development through the ESTHER programme (Ensemble pour une Solidarité Thérapeutique Hospitaliére En Réseau) and by the German Federal Ministry of Education and Research (grant 01DG13006A, MOPACUR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ignatius, R., Klemm, T., Zander, S. et al. Highly specific detection of Cryptosporidium spp. oocysts in human stool samples by undemanding and inexpensive phase contrast microscopy. Parasitol Res 115, 1229–1234 (2016). https://doi.org/10.1007/s00436-015-4859-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4859-3