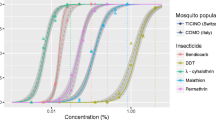
Abstract
Dengue and chikungunya are important arboviral infections in the Andaman Islands. Competent vectors viz. Aedes aegypti and Aedes albopictus are widely prevalent. The most effective proven method for interrupting the transmission of these arboviruses is vector control, mediated through insecticides. Currently, DDT and temephos are the insecticides used for vector control in these islands. Lack of information on susceptibility necessitated assessing the susceptibility profile of A. aegypti and A. albopictus. F1 generation of adult and larvae were assayed, and LT50 and LT90 values were interpreted following the World Health Organization (WHO) protocol. Adults were found resistant to DDT-4 % while susceptible to dieldrin-0.4 %. Against organophosphates, both showed resistance to fenitrothion but susceptible to malathion-5 %. Both species showed resistance to carbamate and bendiocarb-0.1 % while susceptible to propoxur-0.1 %. Of the four synthetic pyrethroids, both were susceptible to deltamethrin-0.05 %, while resistant to permethrin-0.75 %, lambdacyhalothrin-0.05 % and cyfluthrin-0.15 %. Larvae of both species showed resistance to temephos at 0.02 mg/L but susceptible to malathion at 1 mg/L and fenthion at 0.05 mg/L. Currently, there is no prescribed WHO dose for adult-insecticide susceptibility testing. The emergence of resistance to DDT and temephos in the vector population poses a challenge to the on-going vector control measures. The results highlight the need for monitoring resistance to insecticides in the vector population. Impetus for source reduction and alternative choices of control measures are discussed for tackling future threat of arboviral infections in these islands.

Similar content being viewed by others
References
Abbott WS (1925) A method of computing the effectiveness an insecticide. J Econ Entomol 18:265–267
Amalraj D, Sahu SS, Jambulingam P et al (2000) Efficacy of aqueous suspension and granular formulations of Bacillus thuringiensis (Vectobac) against mosquito vectors. Acta Trop 7:243–246
Andaman and Nicobar administration (2009) Statistics book, Directorate of statistics, Port Blair, Andaman and Nicobar administration
Anonymous. Annual Report (2009-10), Regional Medical Research Centre, Port Blair
Bhattacharya A, Barik SR, Ganguly P (2009) New pesticide molecules, formulation technology and uses: present status and future challenges. J Plant Prot Sci 1:9–15
Brogdon WG, McAllister JC (1998) Insecticide resistance and vector control. Emerg Infect Dis 4:605–613
Chaaithanya IK, Bhattacharya D, Muruganandam N et al (2012) Dengue: a newly emerging viral infection in Andaman and Nicobar Islands, India. Epidemiol Infect 140:1920–1924
Chareonviriyaphap T, Bangs MJ, Suwonkerd W et al (2013) Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasit Vectors 25:6–280
Chen CD, Nazni WA, Lee HL, Sofian-Azirun M (2005) Susceptibility of Aedes aegypti and Aedes albopictus to temephos in four study sites in Kuala Lumpur City Center and Selangor State, Malaysia. Trop Biomed 22:207–216
Das BP, Kabilan L, Sharma SN, Lal S, Ragu K, Saxena VK (2004) Detection of dengue virus in wild caught Aedes albopictus (Skuse) around Calicut Airport, Malapuram district, Kerala, India. Dengue Bull 28:210–212
Dash AP, Raghavendra K, Pillai MKK (2006) Combating resistance to insecticides in malaria control—gains made in India. Bayer Environ Sci J 18:30–37
Dhiman S, Rabha B, Yadav K, Baruah I, Veer V (2014) Insecticide susceptibility and dengue vector status of wild Stegomya albopicta in a strategically important area of Assam, India. Parasit Vectors 7:295
Dia I, Diagne CT, Ba Y, Diallo D, Konate L, Diallo M (2012) Insecticide susceptibility of Aedes aegypti populations from Senegal and Cape Verde Archipelago. Parasit Vectors 22:5–238
Dusfour I, Thalmensy V, Gaborit P, Issaly J, Carinci R, Girod R (2011) Multiple insecticide resistance in Aedes aegypti (Diptera: Culicidae) populations compromises the effectiveness of dengue vector control in French Guiana. Mem Inst Oswaldo Cruz 106:346–352
Farajollahi A, Williams GM, Condon GC, Kesavaraju B, Unlu I, Gaugler R (2013) Assessment of a direct application of two Bacillus thuringiensis israelensis formulations for immediate and residual control of Aedes albopictus. J Am Mosq Control Assoc 29:385–388
Ferrari JA (1996) Insecticide resistance In: The biology of disease vectors. Beaty BJ and Marquardt WC (eds). University Press of Colorado, Niwot, Colorado pp: 512–529
Gratz NG (1993) Lessons of Aedes aegypti control in Thailand. Med Vet Entomol 7:1–10
Grisales N, Poupardin R, Gomez S, Fonseca-Gonzalez I, Ranson H, Lenhart A et al (2013) Temephos resistance in Aedes aegypti in Colombia compromises dengue vector control. PLoS Negl Trop Dis 7(9):e2438
Harris AF, Rajatileka S, Ranson H (2010) Pyrethroid. Resistance in Aedes aegypti from Grand Cayman. Am J Trop Med Hyg 83:277–284
Hemingway J, Ranson NH (2000) Insecticide resistance in insect vectors of human disease. Annu Rev Entomol 45:371–391
Hidayati H, Nazni WA, Lee HL, Sofian-Azirun M (2011) Insecticide resistance development in Aedes aegypti upon selection pressure with malathion. Trop Biomed 28:425–437
Jagadeshwaran U, Vijayan VA (2009) Biochemical characterization of deltamethrin resistance in a laboratory-selected strain of Aedes aegypti. Parasitol Res 104:1431–1438
Jirakanjanakit N, Rongnoparut P, Saengtharatip S (2007) Insecticide susceptible/resistance status in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Thailand during 2003–2005. J Econ Entomol 100:545–550
Kamgang B, Marcombe S, Chandre F et al (2011) Insecticide susceptibility of Aedes aegypti and Aedes albopictus in Central Africa. Parasit Vectors 15:4–79
Karunaratne SH, Weeraratne TC, Perera MD, Surendran SN (2013) Insecticide resistance, and efficacy of space spraying and larviciding in the control of dengue vectors Aedes aegypti and Aedes albopictus in Sri Lanka. Pestic Biochem Physiol 107:98–105
Khan HA, Akram W, Shehzad K, Shaalan EA (2011) First report of field evolved resistance to agrochemicals in dengue mosquito, Aedes albopictus (Diptera: Culicidae), from Pakistan. Parasit Vectors 2:4–146
Komalamisra N, Srisawat R, Phanbhuwong T, Oatwaree S (2011) Insecticide susceptibility of the dengue vector, Aedes aegypti (L.) in Metropolitan Bangkok. Southeast Asian J Trop Med Public Health 4:814–823
Krishnamoorthy K, Jambulingam P, Natarajan R et al (2005) Altered environment and risk of malaria outbreak in South Andaman, Andaman & Nicobar Islands, India affected by tsunami disaster. Malar J 20:4–32
Macoris Mde L, Andrighetti MT, Takaku L et al (2003) Resistance of Aedes aegypti from the state of São Paulo, Brazil, to organophosphates insecticides. Mem Inst Oswaldo Cruz 98:703–708
Macoris Mde L, Andrighetti MT, Otrera VC et al (2007) Association of insecticide use and alteration on Aedes aegypti susceptibility status. Mem Inst Oswaldo Cruz 102:895–900
Madhukar BVR, Pillai MKK (1968) Insecticide susceptibility in Indian strains of Aedes aegypti (Linn). Mos News 28:222–225
Manimunda SP, Singh SS, Sugunan AP et al (2007) Chikungunya fever, Andaman and Nicobar Islands, India. Emerg Infect Dis 13:1259–1260
Marina CF, Bond JG, Casas M et al (2011) Spinosad as an effective larvicide for control of Aedes albopictus and Aedes aegypti, vectors of dengue in southern Mexico. Pest Manag Sci 67:114–121
Mukhopadhyay AK, Patnaik SK, Babu PS (2006) Susceptibility status of some culicine mosquitoes to insecticides in Rajahmundry town of Andhra Pradesh, India. J Vector Borne Dis 43:39–41
Mulyatno KC, Yamanaka A, Ngadino KE (2012) Resistance of Aedes aegypti (L.) larvae to temephos in Surabaya, Indonesia. Southeast Asian. J Trop Med Public Health 43:29–33
Muruganandam N, Chaaithanya IK, Senthil GS et al (2011) Isolation and molecular characterization of Chikungunya virus from the Andaman and Nicobar archipelago, India: evidence of an East, Central, and South African genotype. Can J Microbiol 57:1073–1077
Muruganandam N, Chaaithanya IK, Mullaikodi S et al (2014) Dengue virus serotype-3 (subtype-III) in Port Blair, India. J Vector Borne Dis 51:58–61
Paupy C, Delatte H, Bagny L, Corbe V, Fontenille D (2009) Aedes albopictus, an arbovirus vector: from the darkness to the light. Microbes Infect 11:1177–1185
Polson KA, Brogdon WG, Rawlins SC, Chadee DD (2011) Characterization of insecticide resistance in Trinidadian strains of Aedes aegypti mosquitoes. Acta Trop 117:31–38
Raghavan NGS, Wattal BL, Bhatnagar VN, Choudhury DS, Joshi GC, Krishnan KS (1967) Present status of susceptibility of arthropods of public health importance to insecticides in India. Bull Indian Soc Malar Commun Dis 4:209–245
Ritchie SA, Rapley LP, Benjamin S (2010) Bacillus thuringiensis var. israelensis (Bti) provides residual control of Aedes aegypti in small containers. Am J Trop Med Hyg 82:1053–1059
Rong LS, Ann AT, Ahmad NW, Lim LH, Azirun MS (2012) Insecticide susceptibility status of field-collected Aedes (Stegomyia) aegypti (L.) at a dengue endemic site in Shah Alam, Selangor, Malaysia. Southeast Asian J Trop Med Public Health 43:34–47
Shriram AN, Sehgal SC (1999) Aedes aegypti (L) in Port Blair, Andaman and Nicobar islands-distribution and larval ecology. J Commun Dis 31:185–192
Shriram AN, Sugunan AP, Vijayachari P (2008) Infiltration of Aedes aegypti into peri-urban areas in South Andaman. Indian J Med Res 127:618–620
Shriram AN, Sugunan AP, Manimunda SP, Vijayachari P (2009) Community-centred approach for the control of Aedes Spp in a peri-urban zone in the Andaman and Nicobar Islands using Temephos. Natl Med J India 22:116–120
Singh RK, Dhiman RC, Mittal PK, Dua VK (2011) Susceptibility status of dengue vectors against various insecticides in Koderma (Jharkhand) India. J Vector Borne Dis 48:116–118
Somboon P, Prapanthadara LA, Suwonkerd W (2003) Insecticide susceptibility tests of Anopheles minimus, Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus in northern Thailand. Southeast Asian J Trop Med Public Health 34:87–93
Somwang P, Yanola J, Suwan W, Walton C, Lumjuan N, Prapanthadara LA, Somboon P et al (2011) Enzymes-based resistant mechanism in pyrethroid resistant and susceptible Aedes aegypti strains from northern Thailand. Parasitol Res 109:531–537
Sunish IP, Shriram AN, Arun S, Vijayachari P (2014) Spatio-temporal distribution of Aedes mosquitoes in Car Nicobar Island: implication in the transmission of arboviruses. J Asia Pac Entomol 17:761–766
Swarnam TP, Velmurugan A (2013) Pesticide residues in vegetable samples from the Andaman Islands, India. Environ Monit Assess 185:6119–6127
Tan AW, Loke SR, Benjamin S, Lee HL, Chooi KH, Sofian-Azirun M (2012) Spray application of Bacillus thuringiensis israelensis (Bti strain AM65-52) against Aedes aegypti (L.) and Ae. albopictus Skuse populations and impact on dengue transmission in a dengue endemic residential site in Malaysia. Southeast Asian J Trop Med Public Health 43:296–310
Thenmozhi V, Hiryan J, Tiwari SC, Samuel P (2007) Natural and vertical transmission of dengue virus in Aedes albopictus in southern India, state Kerala. Jpn J Infect Dis 60:245–259
Tikar SN, Mendki MJ, Chandel K, Parashar BD, Prakash S (2008) Susceptibility of immature stages of Aedes (Stegomyia) aegypti; vector of dengue and chikungunya to insecticides from India. Parasitol Res 102:907–913
Tikar SN, Kumar A, Prasad GBKS, Prakash S (2009) Temephos-induced resistance in Aedes aegypti and its cross-resistance studies to certain insecticides from India. Parasitol Res 105:57–63
Vijayachari P, Singh SS, Sugunan AP et al (2011) Emergence of dengue in Andaman and Nicobar archipelago: eco-epidemiological perspective. Indian J Med Res 134:235–237
Vythilingam I, Chiang GL, Amatachaya C (1992) Adulticidal effect of cyfluthrin against mosquitos of public health importance in Malaysia. Southeast Asian J Trop Med Public Health 23:111–115
World Health Organization (1981a) Instructions for determining the susceptibility or resistance of adult mosquito to organo-chlorine organophosphate and carbonate insecticides–Diagnostic test. WHO/VBC/81-806
World Health Organization (1981b) Instructions for determining the susceptibility or resistance of mosquito larvae to insecticides. WHO/VBC/81-807
World Health Organization (2005) Guide lines for laboratory and field testing of mosquito larvicides. Geneva
World Health Organization (2012) Handbook for integrated vector management, ISBN: 978 92 4 150280 1 WHO/HTM/NTP/VEM/2012.3
World Health Organization (2013) Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. ISBN: 978 92 4 150515 4
World Health Organization Newsletter (2006) Epidemic and pandemic alert and response, chikungunya and dengue in southwest Indian Ocean. March 2006 Disease outbreak news
Yadav K, Rabha B, Dhiman S, Veer V (2015) Multi-insecticide susceptibility evaluation of dengue vectors Stegomyia albopicta and St. aegypti in Assam, India. Parasit Vectors 8:143
Zeller HG (1998) Dengue, arbovirus and migrations in the Indian Ocean. Bull Soc Pathol Exot 91:56–60
Acknowledgments
The authors are grateful to Dr P Vijayachari, Director, Regional Medical Research Centre (ICMR), Port Blair for extending all the facilities for undertaking this study. Authors acknowledge the useful suggestions on the manuscript provided by Dr K Raghavendra, Scientist “E”, National Institute of Malaria Research (ICMR), New Delhi. Technical Assistance rendered by the staff of the Division of Medical Entomology and Vector Borne Diseases is gratefully acknowledged throughout the conduct of study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivan, A., Shriram, A.N., Sunish, I.P. et al. Studies on insecticide susceptibility of Aedes aegypti (Linn) and Aedes albopictus (Skuse) vectors of dengue and chikungunya in Andaman and Nicobar Islands, India. Parasitol Res 114, 4693–4702 (2015). https://doi.org/10.1007/s00436-015-4717-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-015-4717-3