Abstract
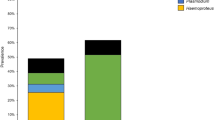
The occurrence of haemosporidians in biting midges of the genus Culicoides is examined in North-East Bulgaria in order to reveal their potential role for parasite transmission. A PCR-based technique amplifying part of the mitochondrial cytochrome b gene of the parasite is applied on naturally infected biting midges. Totally, 640 parous individuals of four species and 95 blood-fed individuals of six species of Culicoides are examined for the presence of DNA of haemosporidians. Haemosporidian genetic lineages are identified in individuals of three insect species: Culicoides alazanicus (12 lineages, nine lineages of Haemoproteus and three lineages of Plasmodium), Culicoides festivipennis and Culicoides circumscriptus (with two and one lineages of Haemoproteus, respectively). Two genetic lineages of Haemoproteus are recorded in more than one vector species. These results demonstrate variations in the specificity of Haemoproteus genetic lineages to their potential vectors, since some lineages are recorded in a single vector species and others occur in two or more vector species.
Similar content being viewed by others
References
Bensch S, Åkesson S (2003) Temporal and spatial variation of hematozoans in Scandinavian willow warblers. J Parasitol 89:388–391
Bensch S, Stjernman M, Hasselquist D, Ostman O, Hansson B, Westerdahl H, Pinheiro RT (2000) Host specificity in avian blood parasites: a study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc R Soc B 267(1452):1583–1589. doi:10.1098/rspb.2000.1181
Bensch S, Pérez-Tris J, Waldenström J, Hellgren O (2004) Linkage between nuclear and mitochondrial DNA sequences in avian malaria parasites: multiple cases of cryptic speciation? J Organ Evol 58:1617–1621
Bensch S, Hellgren O, Pérez-Tris J (2009) MalAvi: a public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol Ecol Resour 9:1353–1358. doi:10.1111/j.1755-0998.2009.02692.x
Bensch S, Jönsson J, Copete JL (2012) Low prevalence of Haemoproteus infections in Chiffchaffs. Parasitology 139:302–309
Bobeva A, Zehtindjiev P, Bensch S, Radrova J (2013) A survey of biting midges of the genus Culicoides Latreille, 1809 (Diptera: Ceratopogonidae) in NE Bulgaria, with respect to transmission of avian haemosporidians. Acta Parasitol 58:585–591. doi:10.2478/s11686-013-0185-z
Bobeva A, Zehtindjiev P, Ilieva M, Dimitrov D, Mathis A, Bensch S (2014) Host preferences of ornithophilic biting midges of the genus Culicoides in Eastern Balkans with respect to transmission of haemosporidian parasites. Med and Vet Entomol, in press
Chakarov N, Boerner M, Krüger O (2008) Fitness in common buzzards at the cross-point of opposite melanin-parasite interactions. Funct Ecol 22:1062–1069. doi:10.1111/j.1365-2435.2008.01460.x
Chvála M, Hrka K, Chalupský J, Knoz J, Miná J, Orszägh I (1980) Krevsající mouchy a střečci. Academia, Praha (in Czech)
Cosgrove CL, Wood MJ, Day KP, Sheldon BC (2008) Seasonal variation in Plasmodium prevalence in a population of blue tits Cyanistes caeruleus. J Anim Ecol 77:540–548
Delécolle JC (1985) Nouvelle contribution à l’étude systématique et iconographique des espèces du genre Culicoides (Diptera: Ceratopogonidae) du Nord-Est de la France. Disertation, Université Louis Pasteur, Strasbourg.
Dimitrov D, Zehtindjiev P, Bensch S (2010) Genetic diversity of avian blood parasites in SE Europe: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Bulgaria. Acta Parasitol 55:201–209. doi:10.2478/s11686-010-0029-z
Dyce AL (1969) The recognition of nulliparous and parous Culicoides (Diptera: Ceratopogonidae) without dissection. Aust J Entomol 8:11–15
Fallon SM, Bermingham E, Ricklefs RE (2005) Host specialization and geographic localization of avian malaria parasites: a regional analysis in the Lesser Antilles. Am Nat 165:466–480. doi:10.1086/428430
Ferraguti M, Martínez-de la Puente J, Ruiz S, Soriguer R, Figuerola J (2013) On the study of the transmission networks of blood parasites from SW Spain: diversity of avian haemosporidians in the biting midge Culicoides circumscriptus and wild birds. Parasitol Vectors. doi:10.1186/1756-3305-6-208
Garnham PCC (1966) Malaria parasites and other Haemosporidia. Blackwell Scientific Publications, Oxford
Glukhova VM (1989) Krovososushchie mokretsy rodov Culicoides i Forcopomiya (Ceratopogonidae). In: Viogradova EB, Kerzhner IM (eds) Fauna SSSR. Izdatel’stvo Nauka, Moscow (in Russian)
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows95/98/NT. Nucleic Acids Symp Ser 41:95–98
Hellgren O, Križanauskiene A, Valkiūnas G, Bensch S (2007) Diversity and phylogeny of mitochondrial cytochrome B lineages from six morphospecies of avian Haemoproteus (Haemosporida: Haemoproteidae). J Parasitol 93(4):889–896
Kaufmann C, Ziegler D, Schaffner F, Carpenter S, Pflüger V, Mathis A (2011) Evaluation of matrix-assisted laser desorption/ionization time of flight mass spectrometry for characterization of Culicoides nubeculosus biting midges. Med and Vet Entomol 25:32–38. doi:10.1111/j.1365-2915.2010.00927.x
Knowles SCL, Palinauskas V, Sheldon BC (2010) Chronic malaria infections increase family inequalities and reduce parental fitness: experimental evidence from a wild bird population. J Evol Biol 23:557–569. doi:10.1111/j.1420-9101.2009.01920.x
Knowles SCL, Wood MJ, Alves R, Wilkin TA, Bensch S, Sheldon BC (2011) Molecular epidemiology of malaria prevalence and parasitaemia in a wild bird population. Mol Ecol 20:1062–1076
Križanauskienė A, Hellgren O, Kosarev V, Sokolov L, Bensch S, Valkiūnas G (2006) Variation in host specificity between species of avian hemosporidian parasites: evidence from parasite morphology and cytochrome b gene sequences. J Parasitol 92(6):1319–1324
Kulma K, Low M, Bensch S, Qvarnström A (2013) Malaria infections reinforce competitive asymmetry between two Ficedula flycatchers in a recent contact zone. Mol Ecol 22:4591–4601
Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon BC (2011) Fitness effects of endemic malaria infections in a wild bird population: the importance of ecological structure. J Anim Ecol 80(6):1196–1206. doi:10.1111/j.1365-2656.2011.01836.x
Laird PW, Zijderveld A, Linders K, Rudnicki MA, Jaenisch R, Berns A (1991) Simplified mammalian DNA isolation procedure. Nucleic Acids Res 19:4293
Lassen SB, Nielsen SA, Kristensen M (2012) Identity and diversity of blood meal hosts of biting midges (Diptera: Ceratopogonidae: Culicoides Latreille) in Denmark. Parasitol Vectors. doi:10.1186/1756-3305-5-143
Martinez-de la Puente J, Merino S, Tomás G, Moreno J, Morales J, Lobato E et al (2010) The blood parasite Haemoproteus reduces survival in a wild bird: a medication experiment. Biol Lett 6:663–665. doi:10.1098/rsbl.2010.0046
Martínez-de la Puente J, Martínez J, Rivero-de Aguilar J, Herrero J, Merino S (2011) On the specificity of avian blood parasites: revealing specific and generalist relationships between haemosporidians and biting midges. Mol Ecol 20:3275–3287. doi:10.1111/j.1365-294X.2011.05136.x
Marzal A, Bensch S, Reviriego M, Balbontin J, De Lope F (2008) Effects of malaria double infection in birds: one plus one is not two. J Evol Biol 21:979–987
Mellor PS, Boorman J, Baylis M (2000) Culicoides biting midges: their role as arbovirus vectors. Annu Rev Entomol 45:307–340
Nedelchev N (2008) Prouchvane varkhu nasekomite ot rod Culicoides (Diptera: Ceratopogonidae) v Balgariya (Habilitation thesis). Sofia, Central Research Diagnostic Veterinary Institute, 343 pp. (in Bulgarian)
Pérez-Tris J, Bensch S (2005) Dispersal increases local transmission of avian malarial parasites. Ecol Lett 8:838–845
Pettersson E, Bensch S, Ander M, Chirico J, Sigvald R, Ignell R (2012) Molecular identification of bloodmeals and species composition in Culicoides biting midges. Med Vet Entomol 27:104–112. doi:10.1111/j.1365-2915.2012.01038.x
Piersma T, van der Velde M (2012) Dutch House Martins Delichon urbicum gain blood parasite infections over their lifetime, but do not seem to suffer. J Ornithol 153:907–912
Remm H (1988) Ceratopogonidae. In: Soos (ed) Catalogue of Palaearctic Diptera. 3. Budapest, Academiai Kiado, pp 11–110
Santiago-Alarcon D, Havelka P, Schaefer HM, Segelbacher G (2012b) Bloodmeal analysis reveals avian infections and broad host preferences of Plasmodium (Diptera: Ceratopogonidae) vectors. PloS One, e31098. doi:10.1371/journal.pone.0031098
Santiago-Alarcon D, Palinauskas V, Schaefer HM (2012b) Diptera vectors of avian Haemosporidian parasites: untangling parasite life cycles and their taxonomy. Biol Rev Camb Philos Soc 87:928–964. doi:10.1111/j.1469-185X.2012.00234.x
Santiago-Alarcon D, Havelka P, Pineda E, Segelbacher G, Schaefer HM (2013) Urban forests as hubs for novel zoonosis: blood meal analysis, seasonal variation in Culicoides (Diptera: Ceratopogonidae) vectors, and avian haemosporidians. Parasitology. doi:10.1017/S0031182013001285
Shurulinkov P, Golemansky V (2002) Haemoproteids (Haemosporida: Haemoproteidae) of wild birds in Bulgaria. Acta Protozool 41:359–374
Shurulinkov P, Golemansky V (2003) Plasmodium and Leucocytozoon (Sporozoa: Haemosporida) of wild birds in Bulgaria. Acta Protozool 41:205–214
Shurulinkov P, Ilieva M (2009) Spatial and temporal differences in the blood parasite fauna of passerine birds during the spring migration in Bulgaria. Parasitol Res 104:1453–1458. doi:10.1007/s00436-009-1349-5
Synek P, Munclinger P, Albrecht T, Votýpka J (2013) Avian haemosporidians in haematophagous insects in the Czech Republic. Parasitol Res 112:839–845. doi:10.1007/s00436-012-3204-3
Szöllősi E, Cichoń M, Eens M, Hasselquist D, Kempenaers B, Merino S, Nilsson JÅ, Rosivall B, Rytkönen S, Török J, Wood MJ, Garamszegi LZ (2011) Determinants of distribution and prevalence of avian malaria in blue tit populations across Europe: separating host and parasite effects. J Evol Biol 24:2014–2024
Valkiūnas G (2005) Avian malaria parasites and other haemosporidia. CRC Press, Boca Raton
Valkiūnas G (2011) Haemosporidian vector research: marriage of molecular and microscopical approaches is essential. Mol Ecol 20:3084–3086. doi:10.1111/j.1365-294X.2011.05187.x
Valkiūnas G, Iezhova TA, Golemansky V, Pilarska D, Zehtindjiev P (1999) Blood protozoan parasites (Protozoa: Kinetoplastida and Haemosporida) in wild birds from Bulgaria. Acta Zool Bulg 51:127–129
Valkiūnas G, Zehtindjiev P, Hellgren O, Ilieva M, Iezhova TA, Bensch S (2007) Linkage between mitochondrial cytochrome b lineages and morphospecies of two avian malaria parasites, with a description of Plasmodium (Novyella) ashfordi sp. nov. Parasitol Res 100:1311–1322. doi:10.1007/s00436-006-0409-3
Valkiūnas G, Zehtindjiev P, Dimitrov D, Križanauskienė A, Iezhova TA, Bensch S (2008a) Polymerase chain reaction-based identification of Plasmodium (Huffia) elongatum, with remarks on species identity of haemosporidian lineages deposited in GenBank. Parasitol Res 102:1185–1193. doi:10.1007/s00436-008-0892-9
Valkiūnas G, Iezhova TA, Križanauskienė A, Palinauskas V, Bensch S (2008b) In vitro hybridization of Haemoproteus spp.: an experimental approach for direct investigation of reproductive isolation of parasites. J Parasitol 94:1385–1394
Valkiūnas G, Palinauskas V, Križanauskienė A, Bernotiene R, Kazlauskiene R, Iezhova TA (2013) Further observations on in vitro hybridization of hemosporidian parasites: patterns of ookinete development in Haemoproteus spp. J Parasitol 99:124–136
van Rooyen J, Lalubin F, Glaizot O, Christe P (2013) Altitudinal variation in haemosporidian parasite distribution in great tit populations. Parasitol Vectors 6:139
Ventim R, Morais J, Pardal S, Mendes L, Ramos JA, Perez-Tris J (2012) Host-parasite associations and host-specificity in haemoparasites of reed bed passerines. Parasitology 139:310–316
Waldenström J, Bensch S, Hasselquist D, Östman Ö (2004) A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J Parasitol 90:191–194
Wenk CE, Kaufmann C, Schaffner F, Mathis A (2012) Molecular characterization of Swiss Ceratopogonidae (Diptera) and evaluation of real-time PCR assays for the identification of Culicoides biting midges. Vet Parasitol 184:258–266. doi:10.1016/j.vetpar.2011.08.034
Wood MJ, Cosgrove CL, Wilkin TA, Knowles SC, Day KP, Sheldon BC (2007) Within-population variation in prevalence and lineage distribution of avian malaria in blue tits, Cyanistes caeruleus. Mol Ecol 16:3263–3273
Zehtindjiev P, Ilieva M, Westerdahl H, Hansson B, Valkiūnas G, Bensch S (2008) Dynamics of parasitemia of malaria parasites in a naturally and experimentally infected migratory songbird, the great read warbler Acrocephalus arundinaceus. Exp Parasitol 119:99–110. doi:10.1016/j.exppara.2007.12.018, the abbreviation cf. (confer) – C. cf. griseidorsum
Zehtindjiev P, Ivanova K, Mariaux J, Georgiev BB (2013) First data on the genetic diversity of avian haemosporidians in China: cytochrome b lineages of the genera Plasmodium and Haemoproteus (Haemosporida) from Gansu Province. Parasitol Res 112:3509–3515. doi:10.1007/s00436-013-3533-x
Zilahi G (1934) Beiträge zur Fliegenfauna Bulgariens. I. Chironomiden. Bull de la Soc Entomol de Bulg 8:152–158
Acknowledgments
This study is report no. 55 of the Kalimok Field Station. We acknowledge Dr. Bruno Mathieu (University of Strasbourg) and Dr. Alexander Mathis (University of Zürich) for their contribution to the identification of Culicoides biting midges. We are grateful to Dr. Staffan Bensch from Lund University, to Dr. Boyko Georgiev from the Institute of Biodiversity and Ecosystem Research—BAS and to Dr. Ognyan Mikov from the National Centre for Infectious and Parasitic Diseases for the valuable suggestions during the course of the present study. The authors are grateful to the staff of Kalimok Field Station, Boyko Neov and Christoffer Sjöholm for their support with the collection of insects and to Nikola Bankov for his help with the laboratory work. Facilities developed in the frames of the projects WETLANET (FP7 CAPACITIES Grant 229802), CEBDER (National Science Fund, Ministry of Education, Youth and Science of the Republic of Bulgaria, Grant DO 02-15/2009) and “Development of scientific potential in the field of faunistic diversity and environment protection” (funded by the Ministry of Education, Youth and Science and the European Social Fund, Operational Program “Human Resources Development”, Grant BG 051 PO001-3.3.04/41) were used in the course of the present study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bobeva, A., Ilieva, M., Dimitrov, D. et al. Degree of associations among vectors of the genus Culicoides (Diptera: Ceratopogonidae) and host bird species with respect to haemosporidian parasites in NE Bulgaria. Parasitol Res 113, 4505–4511 (2014). https://doi.org/10.1007/s00436-014-4140-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4140-1