Abstract
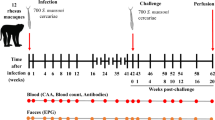
Based on data obtained using vaccine efficacy studies in mice, hamsters, and baboons, the credentials of Sm-p80 as a first tier vaccine candidate for schistosomiasis have been well established. Sm-p80-based vaccine formulation(s) have consistently exhibited potent prophylactic efficacy in reducing adult worm burden following cercarial challenge and induce killing of established adult worms in chronic infection. This vaccine is protective against both intestinal and urinary schistosomiasis. In this study, the longevity of Sm-p80-specific antibody responses was studied in mice and in baboons. Robust antibody titers were detected in mice for up to 60 weeks following vaccination with Sm-p80 recombinant vaccine (Sm-p80 + GLA-SE). In the follow-up experiments to our published studies, Sm-p80-specific IgG was also detected in baboons 5–8 years following the initial vaccination with an Sm-p80 DNA vaccine. In one baboon, transfer of Sm-p80-specific antibody was detected in umbilical cord blood and in the baby. These long-lasting humoral immune response data coupled with the vaccine efficacy data in rodents and nonhuman primates further strengthens the case for Sm-p80 to be moved forward through development leading to human clinical trials.






Similar content being viewed by others
References
Ahmad G, Zhang W, Torben W, Ahrorov A, Damian RT, Wolf RF, White GL, Carey DW, Mwinzi PN, Ganley-Leal L, Kennedy RC, Siddiqui AA (2011) Preclinical prophylactic efficacy testing of Sm-p80-based vaccine in a nonhuman primate model of Schistosoma mansoni infection and immunoglobulin G and E responses to Sm-p80 in Human serum samples from an area where schistosomiasis is endemic. J Infect Dis 204:1437–1449
Ahmad G, Zhang W, Torben W, Damian RT, Wolf RF, White GL, Chavez-Suarez M, Kennedy RC, Siddiqui AA (2009a) Protective and antifecundity effects of Sm-p80-based DNA vaccine formulation against Schistosoma mansoni in a nonhuman primate model. Vaccine 27:2830–2837
Ahmad G, Zhang W, Torben W, Haskins C, Diggs S, Noor Z, Le L, Siddiqui AA (2009b) Prime-boost and recombinant protein vaccination strategies using Sm-p80 protects against Schistosoma mansoni infection in the mouse model to levels previously attainable only by the irradiated cercarial vaccine. Parasitol Res 105:1767–1777
Attallah AM, Ghanem GE, Ismail H, El Waseef AM (2003) Placental and oral delivery of Schistosoma mansoni antigen from infected mothers to their newborns and children. Am J Trop Med Hyg 68:647–651
Beaumier CM, Gillespie PM, Hotez PJ, Bottazzi ME (2013) New vaccines for neglected parasitic diseases and dengue. Transl Res 162:144–155
Bergquist NR, Leonardo LR, Mitchell GF (2005) Vaccine-linked chemotherapy: can schistosomiasis control benefit from an integrated approach? Trends Parasitol 21:112–117
Bergquist R, Al-Sherbiny M, Barakat R, Olds R (2002) Blueprint for schistosomiasis vaccine development. Acta Trop 82:183–192
Carter D, Reed SG (2010) Role of adjuvants in modeling the immune response. Curr Opin HIV AIDS 5:409–413
Clark EH, Silva CJ, Weiss GE, Li S, Padilla C, Crompton PD, Hernandez JN, Branch OH (2012) Plasmodium falciparum malaria in the Peruvian Amazon, a region of low transmission, is associated with immunologic memory. Infect Immun 80:1583–1592
Elgueta R, de Vries VC, Noelle RJ (2010) The immortality of humoral immunity. Immunol Rev 236:139–150
Gabriel S, De BJ, Phiri IK, Masuku M, Riveau G, Schacht AM, Deelder AM, Van Dam GJ, Vercruysse J (2002) Transplacental transfer of schistosomal circulating anodic antigens in cows. Parasite Immunol 24:521–525
Gabriel S, Geldhof P, Phiri IK, Cornillie P, Goddeeris BM, Vercruysse J (2005) Placental transfer of immunoglobulins in cattle infected with Schistosoma mattheei. Vet Immunol Immunopathol 104:265–272
Hotez PJ, Kamath A (2009) Neglected tropical diseases in sub-saharan Africa: review of their prevalence, distribution, and disease burden. PLoS Negl Trop Dis 3:e412
Ireton GC, Reed SG (2013) Adjuvants containing natural and synthetic Toll-like receptor 4 ligands. Expert Rev Vaccines 12:793–807
Jenkins-Holick DS, Kaul TL (2013) Schistosomiasis. Urol Nurs 33:163–170
Karmakar S, Zhang W, Ahmad G, Torben W, Alam MU, Le L, Damian RT, Wolf RF, White GL, Carey DW, Carter D, Reed SG, Siddiqui AA (2014a) Cross-species protection: Schistosoma mansoni Sm-p80 vaccine confers protection against Schistosoma haematobium in hamsters and baboons. Vaccine 32:1296–1303
Karmakar S, Zhang W, Ahmad G, Torben W, Alam MU, Le L, Damian RT, Wolf RF, White GL, Carey DW, Carter D, Reed SG, Siddiqui AA (2014b) Use of an Sm-p80–based therapeutic vaccine to kill established adult schistosome parasites in chronically infected baboons. J Infect Dis. doi:10.1093/infdis/jiu031
King CH (2010) Parasites and poverty: the case of schistosomiasis. Acta Trop 113:95–104
Kitphati R, Pooruk P, Lerdsamran H, Poosuwan S, Louisirirotchanakul S, Auewarakul P, Chokphaibulkit K, Noisumdaeng P, Sawanpanyalert P, Puthavathana P (2009) Kinetics and longevity of antibody response to influenza A H5N1 virus infection in humans. Clin Vaccine Immunol 16:978–981
Komegae EN, Grund LZ, Lopes-Ferreira M, Lima C (2013a) The longevity of Th2 humoral response induced by proteases natterins requires the participation of long-lasting innate-like B cells and plasma cells in spleen. PLoS One 8:e67135
Komegae EN, Grund LZ, Lopes-Ferreira M, Lima C (2013b) TLR2, TLR4 and the MyD88 signaling are crucial for the in vivo generation and the longevity of long-lived antibody-secreting cells. PLoS One 8:e71185
McManus DP, Loukas A (2008) Current status of vaccines for schistosomiasis. Clin Microbiol Rev 21:225–242
Mo AX, Agosti JM, Walson JL, Hall BF, Gordon L (2014) Schistosomiasis elimination strategies and potential role of a vaccine in achieving global health goals. Am J Trop Med Hyg 90:54–60
Novato-Silva E, Gazzinelli G, Colley DG (1992) Immune responses during human Schistosomiasis mansoni. XVIII. Immunologic status of pregnant women and their neonates. Scand J Immunol 35:429–437
Palmeira P, Costa-Carvalho BT, Arslanian C, Pontes GN, Nagao AT, Carneiro-Sampaio MM (2009) Transfer of antibodies across the placenta and in breast milk from mothers on intravenous immunoglobulin. Pediatr Allergy Immunol 20:528–535
Parker M, Allen T (2011) Does mass drug administration for the integrated treatment of neglected tropical diseases really work? Assessing evidence for the control of schistosomiasis and soil-transmitted helminths in Uganda. Health Res Policy Syst 9:3
Peroni DG, Chirumbolo S, Veneri D, Piacentini GL, Tenero L, Vella A, Ortolani R, Raffaelli R, Boner AL (2013) Colostrum-derived B and T cells as an extra-lymphoid compartment of effector cell populations in humans. J Matern Fetal Neonatal Med 26:137–142
Prichard RK, Basanez MG, Boatin BA, McCarthy JS, Garcia HH, Yang GJ, Sripa B, Lustigman S (2012) A research agenda for helminth diseases of humans: intervention for control and elimination. PLoS Negl Trop Dis 6:e1549
Reed SG, Orr MT, Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608
Rollinson D, Knopp S, Levitz S, Stothard JR, Tchuem Tchuente LA, Garba A, Mohammed KA, Schur N, Person B, Colley DG, Utzinger J (2013) Time to set the agenda for schistosomiasis elimination. Acta Trop 128:423–440
Sabarth N, Savidis-Dacho H, Schwendinger MG, Bruhl P, Portsmouth D, Crowe BA, Kistner O, Barrett PN, Kreil TR, Howard MK (2012) A cell culture-derived whole-virus H5N1 vaccine induces long-lasting cross-clade protective immunity in mice which is augmented by a homologous or heterologous booster vaccination. Vaccine 30:5533–5540
Siddiqui AA, Pinkston JR, Quinlin ML, Saeed Q, White GL, Shearer MH, Kennedy RC (2005) Characterization of the immune response to DNA vaccination strategies for schistosomiasis candidate antigen, Sm-p80 in the baboon. Vaccine 23:1451–1456
Skerry CM, Mahon BP (2011) A live, attenuated Bordetella pertussis vaccine provides long-term protection against virulent challenge in a murine model. Clin Vaccine Immunol 18:187–193
Steinhagen F, Kinjo T, Bode C, Klinman DM (2011) TLR-based immune adjuvants. Vaccine 29:3341–3355
Taillardet M, Haffar G, Mondiere P, Asensio MJ, Pleau-Pison T, Burdin N, Defrance T, Genestier L (2010) Toll-like receptor agonists allow generation of long-lasting antipneumococcal humoral immunity in response to a plain polysaccharidic vaccine. J Infect Dis 202:470–479
Terer CC, Bustinduy AL, Magtanong RV, Muhoho N, Mungai PL, Muchiri EM, Kitron U, King CH, Mutuku FM (2013) Evaluation of the health-related quality of life of children in Schistosoma haematobium-endemic Communities in Kenya: a cross-sectional study. PLoS Negl Trop Dis 7:e2106
Torben W, Ahmad G, Zhang W, Nash S, Le L, Karmakar S, Siddiqui AA (2012) Role of antibody dependent cell mediated cytotoxicity (ADCC) in Sm-p80-mediated protection against Schistosoma mansoni. Vaccine 30:6753–6758
Torben W, Ahmad G, Zhang W, Siddiqui AA (2011) Role of antibodies in Sm-p80-mediated protection against Schistosoma mansoni challenge infection in murine and nonhuman primate models. Vaccine 29:2262–2271
Vujanic A, Snibson KJ, Wee JL, Edwards SJ, Pearse MJ, Scheerlinck JP, Sutton P (2012) Long-term antibody and immune memory response induced by pulmonary delivery of the influenza Iscomatrix vaccine. Clin Vaccine Immunol 19:79–83
Zhang W, Ahmad G, Torben W, Noor Z, Le L, Damian RT, Wolf RF, White GL, Chavez-Suarez M, Podesta RB, Kennedy RC, Siddiqui AA (2010) Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine. J Infect Dis 201:1105–1112
Acknowledgments
This work is supported by a grant from the NIAID/NIH (R01AI071223) to Afzal A. Siddiqui; NIH grants (P40RR012317, P40OD010431 and P40OD010988) to Gary L. White and Roman F. Wolf; SBIR/NIH grant (1R43AI103983) to Darrick Carter and Afzal A. Siddiqui; Bill and Melinda Gates Foundation grant (OPP1055855) to Steven G. Reed and Darrick Carter. Snails were provided by the Schistosome Research Reagent Resource Center for distribution by BEI Resources, NIAID, NIH: Schistosoma mansoni, strain NMRI exposed Biomphalaria glabrata, strain NMRI, NR-21962.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, W., Ahmad, G., Le, L. et al. Longevity of Sm-p80-specific antibody responses following vaccination with Sm-p80 vaccine in mice and baboons and transplacental transfer of Sm-p80-specific antibodies in a baboon. Parasitol Res 113, 2239–2250 (2014). https://doi.org/10.1007/s00436-014-3879-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-3879-8