Abstract
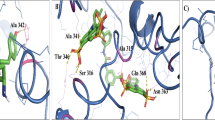
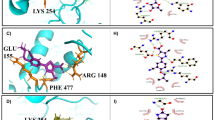
Leishmaniasis is a major health problem worldwide and tools available for their control are limited. Effective vaccines are still lacking, drugs are toxic and expensive, and parasites develop resistance to chemotherapy. In this context, new antimicrobials are urgently needed to control the disease in both human and animal. Here, we report the enzymatic and functional characterization of a Leishmania virulence factor, Leishmania major Protein disulfide isomerase (LmPDI) that could constitute a potential drug target. LmPDI possesses domain structure organization similar to other PDI family members (a, a′, b, b′ and c domains), and it displays the three enzymatic and functional activities specific of PDI family members: isomerase, reductase and chaperone. These results suggest that LmPDI plays a key role in assisting Leishmania protein folding via its capacity to catalyze formation, breakage, and rearrangement of disulfide bonds in nascent polypeptides. Moreover, Bacitracin, a reductase activity inhibitor, and Ribostamycin, a chaperone activity inhibitor, were tested in LmPDI enzymatic assays and versus Leishmania promastigote in vitro cultures and Leishmania amastigote multiplication inside infected THP-1-derived macrophages. Bacitracin inhibited both isomerase and reductase activities, while Ribostamycin had no effect on the chaperone activity. Interestingly, Bacitracin blocked in vitro promastigote growth as well as amastigote multiplication inside macrophages with EC50 values of 39 μM. These results suggest that LmPDI may constitute an interesting target for the development of new anti-Leishmania drugs.



Similar content being viewed by others
Abbreviations
- LmPDI:
-
Leishmania major protein disulfide isomerase
- PDI:
-
Protein disulfide isomerase
References
Ait-Oudhia K, Gazanion E, Vergnes B, Oury B, Sereno D (2011) Leishmania antimony resistance: what we know what we can learn from the field. Parasitol Res 109(5):1225–1232. doi:10.1007/s00436-011-2555-5
Ben Achour Y, Chenik M, Louzir H, Dellagi K (2002) Identification of a disulfide isomerase protein of Leishmania major as a putative virulence factor. Infect Immun 70(7):3576–3585
Ben Achour-Chenik Y, Smith B, Chenik M, Louzir H, Smith DF (2011) Generation and phenotypic analysis of transgenic Leishmania major parasites deleted for the lmpdi virulence gene. Unpublished data
Ben Khalaf N et al (2011) A high-throughput turbidometric assay for screening inhibitors of Leishmania major protein disulfide isomerase. J Biomol Screen 16(5):545–551. doi:10.1177/1087057111401026
Brotherton MC, Racine G, Foucher A, Drummelsmith J, Papadopoulou B, Ouellette M (2010) Analysis of stage-specific expression of basic proteins in Leishmania infantum. J Proteome Res. doi:10.1021/pr100048m
Chenik M, Lakhal S, Ben Khalef N, Zribi L, Louzir H, Dellagi K (2006) Approaches for the identification of potential excreted/secreted proteins of Leishmania major parasites. Parasitology 132(Pt 4):493–509. doi:10.1017/S0031182005009546
Cunningham AC (2002) Parasitic adaptive mechanisms in infection by leishmania. Exp Mol Pathol 72(2):132–141. doi:10.1006/exmp.2002.2418
Handman E (1999) Cell biology of Leishmania. Adv Parasitol 44:1–39
Hatahet F, Ruddock LW (2009) Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal 11(11):2807–2850. doi:10.1089/ARS.2009.2466
Heras B, Shouldice SR, Totsika M, Scanlon MJ, Schembri MA, Martin JL (2009) DSB proteins and bacterial pathogenicity. Nat Rev Microbiol 7(3):215–225. doi:10.1038/nrmicro2087
Holmgren A (1979) Reduction of disulfides by thioredoxin. Exceptional reactivity of insulin and suggested functions of thioredoxin in mechanism of hormone action. J Biol Chem 254(18):9113–9119
Hong BX, Soong L (2008) Identification and enzymatic activities of four protein disulfide isomerase (PDI) isoforms of Leishmania amazonensis. Parasitol Res 102(3):437–446. doi:10.1007/s00436-007-0784-4
Karala AR, Ruddock LW (2010) Bacitracin is not a specific inhibitor of protein disulfide isomerase. FEBS J 277(11):2454–2462. doi:10.1111/j.1742-4658.2010.07660.x
Karamian M, Motazedian MH, Mehrabani D, Gholami K (2007) Leishmania major infection in a patient with visceral leishmaniasis: treatment with Amphotericin B. Parasitol Res 101(5):1431–1434. doi:10.1007/s00436-007-0649-x
Kedzierski L, Sakthianandeswaren A, Curtis JM, Andrews PC, Junk PC, Kedzierska K (2009) Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr Med Chem 16(5):599–614
Krauth-Siegel RL, Comini MA (2008) Redox control in trypanosomatids, parasitic protozoa with trypanothione-based thiol metabolism. Biochim Biophys Acta 1780(11):1236–1248. doi:10.1016/j.bbagen.2008.03.006
Krauth-Siegel RL, Coombs GH (1999) Enzymes of parasite thiol metabolism as drug targets. Parasitol Today 15(10):404–409
Krauth-Siegel RL, Inhoff O (2003) Parasite-specific trypanothione reductase as a drug target molecule. Parasitol Res 90(Suppl 2):S77–S85. doi:10.1007/s00436-002-0771-8
Louzir H et al (1998) Immunologic determinants of disease evolution in localized cutaneous leishmaniasis due to Leishmania major. J Infect Dis 177(6):1687–1695
Martin J, Langer T, Boteva R, Schramel A, Horwich AL, Hartl FU (1991) Chaperonin-mediated protein folding at the surface of groEL through a 'molten globule'-like intermediate. Nature 352(6330):36–42. doi:10.1038/352036a0
McNicoll F et al (2006) A combined proteomic and transcriptomic approach to the study of stage differentiation in Leishmania infantum. Proteomics 6(12):3567–3581. doi:10.1002/pmic.200500853
Morais TR et al (2011) Anti-malarial, anti-trypanosomal, and anti-leishmanial activities of jacaranone isolated from Pentacalia desiderabilis (Vell.) Cuatrec. (Asteraceae). Parasitol Res. doi:10.1007/s00436-011-2454-9
Moreno D, Plano D, Baquedano Y, Jimenez-Ruiz A, Palop JA, Sanmartin C (2011) Antileishmanial activity of imidothiocarbamates and imidoselenocarbamates. Parasitol Res 108(1):233–239. doi:10.1007/s00436-010-2073-x
Mouray E, Moutiez M, Girault S, Sergheraert C, Florent I, Grellier P (2007) Biochemical properties and cellular localization of Plasmodium falciparum protein disulfide isomerase. Biochimie 89(3):337–346. doi:10.1016/j.biochi.2006.11.001
Mussi SV, Fernandes AP, Ferreira LA (2007) Comparative study of the efficacy of formulations containing fluconazole or paromomycin for topical treatment of infections by Leishmania (Leishmania) major and Leishmania (Leishmania) amazonensis. Parasitol Res 100(6):1221–1226. doi:10.1007/s00436-006-0394-6
Paape D, Barrios-Llerena ME, Le Bihan T, Mackay L, Aebischer T (2010) Gel free analysis of the proteome of intracellular Leishmania mexicana. Mol Biochem Parasitol 169(2):108–114. doi:10.1016/j.molbiopara.2009.10.009
Padilla A, Noiva R, Lee N, Mohan KV, Nakhasi HL, Debrabant A (2003) An atypical protein disulfide isomerase from the protozoan parasite Leishmania containing a single thioredoxin-like domain. J Biol Chem 278(3):1872–1878. doi:10.1074/jbc.M210322200
Ramos MA, Mares RE, Magana PD, Ortega JE, Cornejo-Bravo JM (2008) In silico identification of the protein disulfide isomerase family from a protozoan parasite. Comput Biol Chem 32(1):66–70. doi:10.1016/j.compbiolchem.2007.09.002
Santos DO et al (2008) Leishmaniasis treatment—a challenge that remains: a review. Parasitol Res 103(1):1–10. doi:10.1007/s00436-008-0943-2
Santos CX et al (2009) Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J Leukoc Biol 86(4):989–998. doi:10.1189/jlb.0608354
Shakya N, Sane SA, Gupta S (2011) Antileishmanial efficacy of fluconazole and miltefosine in combination with an immunomodulator—picroliv. Parasitol Res 108(4):793–800. doi:10.1007/s00436-010-2230-2
Zhang R et al (2010) In vitro and in vivo antileishmanial efficacy of nitazoxanide against Leishmania donovani. Parasitol Res 107(2):475–479. doi:10.1007/s00436-010-1906-y
Acknowledgements
This work was supported by the Sandler Foundation. We thank Farah Boubaker and Sima Drini for research assistance and Yosser Ben Achour Chenik for English proofreading.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
Supplemental data 1
(PPT 112 kb)
Supplemental data 2
(PPT 227 kb)
Supplemental data 3
(PPT 131 kb)
Supplemental data 4
(PPT 127 kb)
Rights and permissions
About this article
Cite this article
Ben Khalaf, N., De Muylder, G., Louzir, H. et al. Leishmania major protein disulfide isomerase as a drug target. Parasitol Res 110, 1911–1917 (2012). https://doi.org/10.1007/s00436-011-2717-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-011-2717-5