Abstract
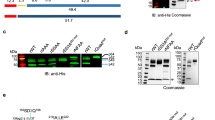
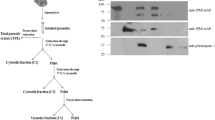
Although ATP-dependent caseinolytic protease (Clp) complexes are important for regulating the pathogenicity, survival, and development of many pathogens, their physiological roles in the pathogenicity of malarial parasites remain unknown. This study reports the cloning, authentication, and characterization of a putative Clp protease subunit from Plasmodium falciparum (PfClpP). Heterologous expression studies showed that signal peptide hindered the soluble expression of the full-length PfClpP. Biochemical analyses of the recombinant PfClpP showed that it did not cleave the known ClpP substrate, succinyl-leucine-tyrosine-7-amido-4-methylcoumarin hydrochloride (AMC). Instead, PfClpP readily hydrolyzed a different substrate, glycine-arginine-AMC. The distinctive substrate preference of PfClpP suggests structural uniqueness in its substrate-binding sites that might be exploitable in anti-malarial drug development. Whether PfClpP resembles most eukaryotic ClpPs in being localized to the mitochondria and chloroplasts was also investigated using a mammalian surrogate host system. The results observed showed that green-fluorescence protein tagged PfClpP proteins were localized to the nucleus. PfClpP may have a unique and specialized role in the plasmodial nucleus. Taken together, this study has shown that PfClpP has a unique peptide cleavage function that is localized at the plasmodial nucleus, probably positioned to elicit a regulatory role in the parasite’s pathogenicity.




Similar content being viewed by others
References
Acharya P, Kumar R, Tatu U (2007) Chaperoning a cellular upheaval in malaria: Heat shock proteins in Plasmodium falciparum. Mol Biochem Parasitol 153(2):85–94
Adam Z, Rudella A, van Wijk KJ (2006) Recent advances in the study of Clp, FtsH and other proteases located in chloroplasts. Curr Opin Plant Biol 9(3):234–240
Andresen BS, Corydon TJ, Wilsbech M, Bross P, Schroeder LD, Hindkjaer TF, Bolund L, Gregersen N (2000) Characterization of mouse Clpp protease cDNA, gene, and protein. Mamm Genome 4:275–280
Arribas J, Castano JG (1993) A comparative study of the chymotrypsin-like activity of the rat liver multicatalytic proteinase and the ClpP from Escherichia coli. J Biol Chem 268(28):21165–21171
Becker K, Tilley L, Vennerstrom JL, Roberts D, Rogerson S, Ginsburg H (2004) Oxidative stress in malaria parasite-infected erythrocytes: host–parasite interactions. Int J Parasitol 34(2):163–189
Bender A, van Dooren GG, Ralph SA, McFadden GI, Schneider G (2003) Properties and prediction of mitochondrial transit peptides from Plasmodium falciparum. Mol Biochem Parasitol 132:59–66
Bozdech Z, Llinas M, Pulliam BL, Wong ED, Zhu J, DeRisi JL (2003) The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol 1(1):E5
Chan M, Tan DS, Wong SH, Sim TS (2006) A relevant in vitro eukaryotic live-cell system for the evaluation of plasmodial protein localization. Biochimie 88(10):1367–1375
Chandu D, Nandi D (2004) Comparative genomics and functional roles of the ATP-dependent proteases Lon and Clp during cytosolic protein degradation. Res Microbiol 155(9):710–719
Chwieralski CE, Welte T, Buhling F (2006) Cathepsin-regulated apoptosis. Apoptosis 11(2):143–149
Cohn MT, Ingmer H, Mulholland F, Jørgensen K, Wells JM, Brøndsted L (2007) Contribution of conserved ATP-dependent proteases of Campylobacter jejuni to stress tolerance and virulence. Appl Environ Microbiol 73(24):7803–7813
Cokol M, Nair R, Rost B (2000) Finding nuclear localization signals. EMBO Rep 1(5):411–415
Corydon TJ, Bross P, Holst HU, Neve S, Kristiansen K, Gregersen N, Bolund L (1998) A human homologue of Escherichia coli ClpP caseinolytic protease: recombinant expression, intracellular processing and subcellular localization. Biochem J 331(Pt 1):309–316
Foth BJ, Ralph SA, Tonkin CJ, Struck NS, Fraunholz M, Roos DS, Cowman AF, McFadden GI (2003) Dissecting apicoplast targeting in the malaria parasite Plasmodium falciparum. Science 299(5607):705–708
Gaillot O, Pellegrini E, Bregenholt S, Nair S, Berche P (2000) The ClpP serine protease is essential for the intracellular parasitism and virulence of Listeria monocytogenes. Mol Microbiol 35(6):1286–1294
Gantt SM, Myung JM, Briones MR, Li WD, Corey EJ, Omura S, Nussenzweig V, Sinnis P (1998) Proteasome inhibitors block development of Plasmodium spp. Antimicrob Agents Chemother 42(10):2731–2738
Gerth U, Kock H, Kusters I, Michalik S, Switzer RL, Hecker M (2008) Clp-dependent proteolysis down-regulates central metabolic pathways in glucose-starved Bacillus subtilis. J Bacteriol 190(1):321–331
Goh LL, Barkham T, Sim TS (2005) Molecular cloning and functional characterization of fumarases C in Neisseria species. Antonie van Leeuwenhoek 87(3):205–213
Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-Pdb Viewer: an environment for comparative protein modeling. Electrophoresis 18(15):2714–2723
Halperin T, Zheng B, Itzhaki H, Clarke AK, Adam Z (2001) Plant mitochondria contain proteolytic and regulatory subunits of the ATP-dependent Clp protease. Plant Mol Biol 45(4):461–468
Hatabu T, Hagiwara M, Taguchi N, Kiyozawa M, Suzuki M, Kano S, Sato K (2006) Plasmodium falciparum: the fungal metabolite gliotoxin inhibits proteasome proteolytic activity and exerts a plasmodicidal effect on P. falciparum. Exp Parasitol 112(3):179–183
Haynes CM, Petrova K, Benedetti C, Yang Y, Ron D (2007) ClpP mediates activation of a mitochondrial unfolded protein response in C. elegans. Dev Cell 13(4):467–480
Jenal U, Fuchs T (1998) An essential protease involved in bacterial cell-cycle control. EMBO J 17(19):5658–5669
Kang SG, Ortega J, Singh SK, Wang N, Huang NN, Steven AC, Maurizi MR (2002) Functional proteolytic complexes of the human mitochondrial ATP-dependent protease, hClpXP. J Biol Chem 277(23):21095–21102
Kang SG, Maurizi MR, Thompson M, Mueser T, Ahvazi B (2004) Crystallography and mutagenesis point to an essential role for the N-terminus of human mitochondrial ClpP. J Struct Biol 148(3):338–352
Kreidenweiss A, Kremsner PG, Mordmüller B (2008) Comprehensive study of proteasome inhibitors against Plasmodium falciparum laboratory strains and field isolates from Gabon. Malar J 7:187
Kwon HY, Kim SW, Choi MH, Ogunniyi AD, Paton JC, Park SH, Pyo SN, Rhee DK (2003) Effect of heat shock and mutations in ClpL and ClpP on virulence gene expression in Streptococcus pneumoniae. Infect Immun 71(7):3757–3765
Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L (1997) Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol 17(3):1129–1143
Maurizi MR, Clark WP, Katayama Y, Rudikoff S, Pumphrey J, Bowers B, Gottesman S (1990) Sequence and structure of Clp P, the proteolytic component of the ATP-dependent Clp protease of Escherichia coli. J Biol Chem 265(21):12536–12545
Mordmüller B, Fendel R, Kreidenweiss A, Gille C, Hurwitz R, Metzger WG, Kun JF, Lamkemeyer T, Nordheim A, Kremsner PG (2006) Plasmodia express two threonine-peptidase complexes during asexual development. Mol Biochem Parasitol 148(1):79–85
Peltier JB, Ytterberg J, Liberles DA, Roepstorffm P, van Wijk KJ (2001) Identification of a 350-kDa ClpP protease complex with 10 different Clp isoforms in chloroplasts of Arabidopsis thaliana. J Biol Chem 276(19):16318–16327
Schweder T, Lee KH, Lomovskaya O, Matin A (1996) Regulation of Escherichia coli starvation sigma factor (sigma s) by ClpXP protease. J Bacteriol 178(2):470–476
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22(22):4673–4680
Vedadi M, Lew J, Artz J, Amani M, Zhao Y, Dong A, Wasney GA, Gao M, Hills T, Brokx S, Qiu W, Sharma S, Diassiti A, Alam Z, Melone M, Mulichak A, Wernimont A, Bray J, Loppnau P, Plotnikova O, Newberry K, Sundararajan E, Houston S, Walker J, Tempel W, Bochkarev A, Kozieradzki I, Edwards A, Arrowsmith C, Roos D, Kain K, Hui R (2007) Genome-scale protein expression and structural biology of Plasmodium falciparum and related Apicomplexan organisms. Mol Biochem Parasitol 151(1):100–110
Wang J, Hartling JA, Flanagan JM (1997) The structure of ClpP at 2.3 Å resolution suggests a model for ATP-dependent proteolysis. Cell 91(4):447–456
Woo KM, Chung WJ, Ha DB, Goldberg AL, Chung CH (1989) Protease Ti from Escherichia coli requires ATP hydrolysis for protein breakdown but not for hydrolysis of small peptides. J Biol Chem 264(4):2088–2091
Yu AY, Houry WA (2007) ClpP: a distinctive family of cylindrical energy-dependent serine proteases. FEBS Lett 581(19):3749–3757
Zheng B, MacDonald TM, Sutinen S, Hurry V, Clarke AK (2006) A nuclear-encoded ClpP subunit of the chloroplast ATP-dependent Clp protease is essential for early development in Arabidopsis thaliana. Planta 224(5):1103–1115
Zuegge J, Ralph S, Schmuker M, McFadden GI, Schneider G (2001) Deciphering apicoplast targeting signals-feature extraction from nuclear-encoded precursors of Plasmodium falciparum apicoplast proteins. Gene 280:19–26
Author information
Authors and Affiliations
Corresponding author
Additional information
This work is funded by an A*STAR Biomedical Research Council grant administered by the National University of Singapore.
Rights and permissions
About this article
Cite this article
Lin, W., Chan, M. & Sim, TS. Atypical caseinolytic protease homolog from Plasmodium falciparum possesses unusual substrate preference and a functional nuclear localization signal. Parasitol Res 105, 1715–1722 (2009). https://doi.org/10.1007/s00436-009-1612-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1612-9