Abstract
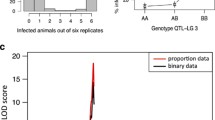
In this study, we investigated the interaction between host outcrossing and infection in the Biomphalaria glabrata–Schistosoma mansoni system. Snails collected from three susceptible isofemale lines were mated with either siblings or snails recently derived from a field site in Brazil. Resulting inbred and outcrossed progeny were then exposed to S. mansoni larvae and monitored for a 10-week period. Interestingly, all snails exhibited equal susceptibility whether they were the result of inbreeding or outcrossing. However, further examination of both host and parasite life-history traits uncovered significant differences between the groups. In uninfected snails, outcrossed progeny tended to exhibit greater fitness relative to inbred progeny. When snails were parasitized, these differences were magnified in certain life-history traits, particularly host reproduction and survival. As an extension of the work, we also investigated virulence within this host–parasite system. Estimates of parasite reproduction and host size were combined to generate a novel “exploitation index,” and these indices were regressed with host survivorship. As predicted, there was a significant and negative correlation between the variables, but this was restricted to a single snail line. Results from this study demonstrate that infection outcomes (as measured by prevalence) may not differ between inbred and outcrossed hosts. However, outcrossing may enhance snail fitness through life-history trait expression.




Similar content being viewed by others
References
Acevedo-Whitehouse K, Gulland F, Greig D, Amos W (2003) Disease susceptibility in California sea lions. Nature 405:679–681
Anderson RM, May RM (1979) Population biology of infectious disease: Part I. Nature 280:361–367
Bean K, Amos W, Pomeroy PP, Twiss SD, Coulson TN, Boyd IL (2004) Patterns of parental relatedness and pup survival in the grey seal (Halichoerus grypus). Mol Ecol 13:2365–2370
Blanford S, Thomas MB, Pugh C, Pell JK (2003) Temperature checks the Red Queen? Resistance and virulence in a fluctuating environment. Ecol Lett 6:2–5
Bull JJ (1994) Perspective: virulence. Evolution 48:1423–1437
Calleri DV, Reid EM, Rosengaus RB, Vargo EL, Traniello JFA (2006) Inbreeding and disease resistance in a social insect: effects of heterozygosity on immunocompetence in the termite Zootermopsis angusticollis. Proc R Soc Lond, B Biol Sci 273:2633–2640
Charbonnel N, Angers B, Rasatavonjizay R, Bremond P, Jarne P (2002) Evolutionary aspects of the metapopulation dynamics of Biomphalaria pfeifferi, the intermediate host for Schistosoma mansoni. J Evol Biol 15:248–261
Coltman DW, Pilkington JG, Smith JA, Pemberton JM (1999) Parasite-mediated selection against inbred soay sheep in a free-living island population. Evolution 53:1259–1267
de Roode JC, Culleton R, Cheeseman SJ, Carter R, Read AF (2004) Host heterogeneity is a determinant of competitive exclusion or coexistence in genetically diverse malaria infections. Proc R Soc Lond, B Biol Sci 271:S104–S106
Dikkeboom R, Van Der Knaap WPW, Meuleman E, Sminia T (1984) Differences between blood cells of juvenile and adult specimens of pond snail Lymnaea stagnalis. Cell Tiss Res 238:43–47
Gandon S, Capowiez Y, Dubois Y, Michalakis Y, Olivieri I (1996) Local adaptation and gene-for-gene coevolution in a metapopulation model. Proc R Soc Lond, B Biol Sci 263:1003–1009
Gow JL, Noble LR, Rollinson D, Mimpfoundi R, Jones CS (2004) Breeding system and demography shape population genetic structure across ecological and climactic zones in the African freshwater snail, Bulinus forskalii (Gastropoda, Pulmonata), intermediate host for schistosomes. Mol Ecol 13:3561–3573
Gower C, Webster JP (2005) Competition and the evolution of virulence in a parasitic trematode. Evolution 59:544–553
Jokela J, Lively CM, Taskinen J, Peters AD (1999) Effect of starvation on parasite-induced mortality in a freshwater snail (Potamopyrgus antipodarum). Oecologia 119:320–325
Jokela J, Taskinen J, Mutikainen P, Kopp K (2005) Virulence of parasites in hosts under environmental stress: experiments with anoxia and starvation. Oikos 108:156–164
Lively CM, Dybdahl ME (2000) Parasite adaptation to locally common host genotypes. Nature 405:679–681
Meagher SA (1999) Genetic diversity and Capillaria hepatica (Nematoda) prevalence in Michigan deer mouse populations. Evolution 53:1318–1324
Poulin R, Combes C (1999) The concept of virulence: Interpretations and implications. Parasitol Today 15:474–475
Rigby MC, Jokela J (2000) Predator avoidance and immune defence: costs and trade-offs in snails. Proc R Soc Lond, B Biol Sci 267:171–176
Rodgers JK, Sandland GJ, Joyce SR, Minchella DJ (2005) Multi-species interactions among a commensal (Chaetogaster limnaei limnaei), a parasite (Schistosoma mansoni), and an aquatic snail host (Biomphalaria glabrata). J Parasitol 91:709–712
Sandland GJ, Carmosini N (2006) Combined effects of an herbicide (atrazine) and predation on the life history of a pond snail, Physa gyrina. Environ Toxicol Chem 25:2216–2220
Sandland GJ, Minchella DJ (2004) Life-history plasticity in hosts (Lymnaea elodes) exposed to differing resources and parasitism. Can J Zool 82:1672–1677
Sandland GJ, Foster AV, Zavodna M, Minchella DJ (2007) Interplay between host genetic variation and parasite transmission in the Biomphalaria glabrata–Schistosoma mansoni system. Parasitol Res 101:1083–1089
Sapp KK, Loker ES (2000) A comparative study of mechanisms underlying digenean-snail specificity: in vitro interactions between hemocytes and digenean larvae. J Parasitol 86:1020–1029
Spielman D, Brook BW, Briscoe DA, Frankham R (2004) Does inbreeding and loss of genetic diversity decrease disease resistance? Conserv Gen 5:439–448
Trouve S, Degen L, Goudet J (2005) Ecological components and evolution of selfing in the freshwater snails, Galba truncatula. J Evol Biol 18:358–370
Valsecchi E, Amos W, Raga JA, Podesta M, Sherwin W (2004) The effects of inbreeding on mortality during a morbillivirus outbreak in the Mediterranean striped dolphin (Stenella coeruleoalba). Anim Conserv 7:139–146
van der Knaap WPW, Meuleman EA, Sminia T (1987) Alterations in the internal defense system of the pond snail Lymnaea stagnalis induced by infection with the schistosome Trichobilharzia ocellata. Parasitol Res 73:57–65
Wethington AR, Zavodna M, Smith MK, Oliveira G, Lewis F, Minchella DJ (2007) Population genetic structure of Biomphalaria glabrata in a schistosomiasis-endemic region in Brazil. J Molluscan Stud 73:45–52
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandland, G.J., Wethington, A.R., Foster, A.V. et al. Effects of host outcrossing on the interaction between an aquatic snail and its locally adapted parasite. Parasitol Res 105, 555–561 (2009). https://doi.org/10.1007/s00436-009-1428-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-009-1428-7