Abstract
The present study is the first investigation on ectoparasites of commercial important fish from Segara Anakan, a brackish water lagoon located at the southern coast of Java, Indonesia. Eight economically important marine fish species (Mugil cephalus, Siganus javus, Scatophagus argus, Caranx sexfasciatus, Lutjanus johnii, Eleutheronema tetradactylum, Johnius coitor, and Epinephelus coioides) were examined for crustacean parasites. Prevalence and intensity data for each parasite species are given, together with an analysis of the origin and possible transmission pathways. A highly divers copepod fauna consisting of 23 different species and two isopods was found. All fish species were at least infested with two copepod species, with the exception of L. johnii, S. argus, and M. cephalus. With seven and six species, respectively, they harboured the most species-rich ectoparasite fauna. The copepods Ergasilus sp. 3 and Caligus acanthopagri on S. argus showed the highest prevalence (78.6) and intensity [17.8 (1–233) and 5.3 (1–22)] of infestation. The recorded parasite fauna is represented by marine, brackish water, and probably also freshwater components. The brackish water environment of Segara Anakan does not prevent disease outbreaks due to parasitic copepods by preventing pathogenic marine or freshwater species to enter the lagoon. This might cause fish health problems if the Segara Anakan Lagoon would be developed for finfish mariculture in future.
Similar content being viewed by others
Introduction
Indonesia is the largest archipelago in the world, consisting of more than 17,000 islands spread on 5.8 million km2 of the sea (Harris 2000). With a coastline of about 81,000 km, Indonesia has the second largest coastal zone in the world. Approximately 830,000 ha of this area are potentially useful for brackish water aquaculture development and more than 140,000 ha are suitable for mariculture cages (Pollunin 1983; Harris 2000). The Segara Anakan area is a brackish water lagoon of approximately 4,000 ha, which is located on the western side of Cilacap (southern coast of Java) and is surrounded by about 14,000 ha of mangrove forests (Naamin 1991). This lagoon plays an important role as a nursery ground, and also has a major ecological function to support a large and productive mangrove ecosystem (Romimohtarto et al. 1991). Around 45 fish species were reported to occur in the Segara Anakan Lagoon (White et al. 1989), but the real species number is probably much higher (Dudley 2000). From 45 species, 10 are commonly caught and reported as economic important species in this area (Naamin 1991).
Indonesia can be considered as the center of marine biodiversity. For example, 23% of the so far described 254 fish parasitic trypanorhynch cestodes have been recorded from Indonesian waters (Palm 2004). Jakob and Palm (2006) recorded 38 fish parasite species from mainly deep-water fish species from Pelabuhan Ratu, southern Java coast. The knowledge on the Indonesian marine fish parasites, however, is still scarce (Palm 2000). In Indonesia, more than 400 different parasite species have been recorded from around 240 marine fish species so far (also see Jakob and Palm 2006). This is only a fraction of the expected fish parasite biodiversity in approximately 3,000 marine fish species in Indonesia, underlining the poor exploration with respect to the occurrence of marine fish parasites (Hutomo 1986). Information from brackish water environments such as the Segara Anakan Lagoon is completely missing. This, nowadays, has become a major problem in identification and treatment of parasites and diseases in the rapidly developing mariculture industry (Sugama 1999; Zafran et al. 2000; Hartono et al. 2001; Roza et al. 2002; Dewi et al. 2003). There is an urgent need to better describe and identify the Indonesian fish parasite fauna to prevent and overcome future fish disease and parasite outbreaks.
The establishment of future mariculture facilities within and in the surrounding of the Segara Anakan Lagoon needs information on potential threats by fish parasites. The present study is the first investigation on ectoparasites from commercial important fish species from Segara Anakan. It contributes to our knowledge on fish parasitic crustaceans on free-living marine fish species from the southern Java coast, which might also be cultivated in Indonesia in future. Because fish parasites are one of the large health problems within mariculture facilities, parasite identification is provided to facilitate the best treatment and more detailed investigation in the future.
Materials and methods
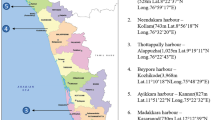
Samples were taken from August to November 2004 at two different localities in the Segara Anakan Lagoon (Central Java). Both selected sampling sites (Motean and Klaces/area 2 and Donan/area 3) are part of the sampling areas of the project “Science for the Protection of Indonesian Coastal Ecosystems” (SPICE) within the German–Indonesian cooperation in Marine Sciences (Fig. 1). They are differentiated by its environmental characteristics. Area 2 is located in the center of the lagoon and is influenced by several rivers that carry freshwater runoff and sedimentation. The salinity is low (19.7–28.0). Area 3 is located near to an outlet to the Indian Ocean. Therefore, the salinity is higher (29.3–31.2).
The fish was obtained directly from local fishermen. The following marine and brackish water fish species were studied from the two sampling sites within Segara Anakan Lagoon: Mugil cephalus L. (70), Scatophagus argus (L., 1766) (70), Siganus javus (L., 1766) (5), Caranx sexfasciatus Quoy and Gaimard, 1825 (8), Lutjanus johnii (Bloch, 1792) (8), Eleutheronema tetradactylum (Shaw, 1804) (8), Johnius coitor (Hamilton, 1822) (20), and Epinephelus coioides (Hamilton, 1822) (21).
Fish dissection was done in the Parasitology and Entomology Laboratory of the Biology Faculty, Jenderal Soedirman University (UNSOED), Purwokerto. Before examination for parasites, the unfrozen fish were measured for length and weight. Each fish was examined microscopically for the presence of parasitic crustaceans based on a method described by Kabata (1985). The collection and preservation methodology for crustacean parasites followed Pritchard and Kruse (1982). Pictures were taken by using a digital camera, Canon PC 1015, attached to the Axioskop 40, and in some cases to a stereomicroscope (STEMI SV 11 Zeiss, Germany). The ecological terms in parasitology, prevalence, intensity, and mean intensity, follow Bush et al. (1997).
Selected specimens were prepared following Robinson et al. (1985) for scanning electron microscopical studies (Fig. 2). A LEITZ-AMR 1000 and International Scientific Instruments ISI-100 B were used for scanning the samples at 30 and 15 kV. The scanning electron microscopy photomicrographs were made with the help of a Leitz, LEICA MD-2, Canada and reflex camera with AGFA APX 25 professional 135, Ilford FP4 plus 125 and Ilford Panf plus 50.
Results
During the present study, 210 fishes (eight species belonging to eight families) from the Segara Anakan Lagoon, central Java, were investigated for the presence of parasitic crustaceans. The isolated parasites belonged to various copepod and two isopod families. All fish species, with the exception of L. johnii, were at least infested with two crustacean species.
Out of all studied fish specimens, only 10 were found uninfested. Copepods were the most common ectoparasites and occurred in nearly all fish samples. In contrast, only two fish species were infested with Isopoda.
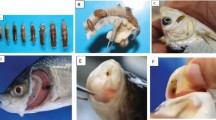
A total of 23 parasitic copepod (Figs. 2, 3, and 4) and two isopod species were found, 23 species occurred as adult and three species were found in the larval stage (Table 1). The copepods of the families Ergasilidae and Caligidae were the most common parasites on the examined fish. M. cephalus and S. argus harbored the most diverse crustacean parasite fauna. A brief description of the collected crustaceans from this unique environment together with notes on the species identification is given below.
Caligid copepod species from Segara Anakan. a Caligus phipsoni (♀) with egg sacs from Eleutheronema tetradactylum; b Caligus sp. (♀) with egg sacs from Johnius coitor; c Caligus cf. confusus (♀) with egg sacs from Caranx sexfasciatus; d Caligus cf. quadratus (♀) with egg sacs from Siganus javus; e Caligus acanthopagri (♀) with egg sacs from Scatophagus argus; f Caligus cf. epinepheli (♀) from Epinephelus coioides; g Caligus rotundigenitalis (♂) from Mugil cephalus; h Caligus epidemicus (♀) from S. argus; i Parapetalus hirsutus (♀) from E. tetradactylum; j Chalimus stage. Scale bars: a and d 1.2 mm; b, e, and g 0.55 mm; c 0.8 mm; f 0.65 mm; h 0.5 mm; i 1.5 mm; j 0.3 mm
Copepod species from Segara Anakan. a Nothobomolochus sp. (♀) with egg sacs from Mugil cephalus; b Ergasilus sp. 1 (♀) with egg sacs from M. cephalus; c Ergasilidae gen. et sp. indet. (♀) from M. cephalus; d Ergasilus sp. 4 (♀) with egg sacs from Siganus javus; e Thysanote sp. (♀) with egg sacs from Scatophagus argus; f Peniculus cf. scomberi (♀) from Caranx sexfasciatus; g Pennellidae gen. et sp. indet. from Epinephelus coioides; h Naobranchia cf. polynemi (♀) from Eleutheronema tetradactylum. Scale bars: a–c 300 μm; d 70 μm; e 1.5 μm; f 1 mm; g 200 μm; h 50 μm
Subclass: Copepoda Milne Edwards, 1830
Order: Cyclopoida Sars, 1886
Family: Bomolochidae Sumpf, 1871
Only a single bomolochid copepod species was collected from the gill mucus of M. cephalus (Fig. 4a). Nothobomolochus sp. is characterized by the structure of the antenna (three modified setae on the proximal segment of antennule) and the number of setae in caudal rami (one major setae). The species was identified after the genus description of Kabata (1979). Many of the 34 species of Nothobomolochus appear remarkably similar, and these similarities have resulted in great taxonomic difficulties. None of these tiny bomolochids is easy to identify. Body shapes and even proportions vary with swelling due to the state of maturity.
Family: Ergasilidae von Nordmann, 1832
A total of five ergasilid species were collected (Fig. 4b–d). The species were identified by using the identification key to ergasilid genera by Boxhall and Halsey (2004). Ergasilus sp. 1 was collected from M. cephalus (Fig. 4b). It is characterized by a typical cyclopiform body shape with clear external segmentation, the presence of biramous fourth legs, antenna with one claw, and the first swimming legs without modified endopod. Ergasilus sp. 2 was collected from S. argus at high prevalence and intensity and from M. cephalus at low prevalence and intensity of infestation. This species differs from Ergasilus sp. 1 in several characters, such as the rounded cephalothorax, the structure and smaller size of the second antenna, and differences in the morphology of the caudal rami, which are not biramous in the present species. Ergasilus sp. 3 was collected from S. argus. It differs from the previous ergasilid species by having a more rounded body shape, differences in caudal rami, and the fine structure of the second antenna. Ergasilus sp. 4 was found at a low prevalence and intensity on S. javus (Fig. 4d). This species differs from the other ergasilids in its body shape and size (shape of thoracic segments, caudal branches, and number of seta.), the morphology of caudal rami and extremely long egg sacs. The morphology of all four species did not correspond to any other known Ergasilus species; the species descriptions will be a matter of another communication.
Ergasilidae gen. et sp. indet. (Fig. 4c)
The genus Ergasilus contains 135 tentatively valid species. Some additional species are treated as species inquirendae or incertae sedis. This species-rich genus lacks revision, making taxonomic work very difficult. In addition, several species have not been well described or not seen again since the original description. In many cases, the type specimens are either inaccessible or not longer extant (Lin and Ho 1998).
Order: Siphonostomatoida Latreille, 1829
Family: Caligidae Burmeister, 1835
A total of 10 caligid species were collected (Fig. 3). Out of these, eight species belong to the genus Caligus, one species to Parapetalus, and one species to Pseudocaligus. Caligus acanthopagri Lin, Ho, Shen, 1994 is characterized by a two-segmented antennule, 3-segmented antenna, a small abdomen, and long egg sacs that are longer than one-half of the body length. C. acanthopagri was collected from the gills and opercula of S. argus at a high prevalence and intensity (Fig. 2). The species was identified by using the original description of Lin et al. (1994). Caligus cf. confusus Pillai, 1961 was collected from C. sexfasciatus. It is characterized by a suborbicular cephalothorax shield, short and 1-segmented abdomen, 3-segmented antenna, and egg sacs shorter than the body. The species is most similar to C. confusus described by Pillai (1961), but showed slight differences in the armature of the antenna and the swimming legs. Caligus epidemicus Hewitt, 1971 was collected from the skin of S. argus and S. javus. This species is characterized by a subcircular cephalothorax shield, a small and 1-segmented abdomen, short caudal rami, 3-segmented antenna, and a subquadrate sternal furca. C. epidemicus was identified by using Ho and Lin (2004). Caligus cf. epinepheli Yamaguti, 1936 was found on the gill filaments of Epinephelus coiodes. This species shared most common features with C. epinepheli, e.g., a subcircular cephalothorax shield, a long abdomen, and 3-segmented antenna. However, the specimens from Segara Anakan showed slight differences in the antenna and swimming legs. Caligus phipsoni Bassett-Smith, 1898 was isolated from the gills of E. tetradactylum. The structure of the first antenna and swimming legs of the present specimen are similar to those in the original description of C. phipsoni. As another important character, C. phipsoni has bigger lunulae than any other Caligus species. Caligus cf. quadratus Shiino, 1954 was found on S. javus. It is characterized by a suborbicular cephalothorax shield, a long abdomen, caudal rami ration 1.6 times longer than wide, and a 3-segmented antenna, but showed slight differences in the armature of the antenna and the swimming legs. The species was identified after Ho and Lin (2004). Caligus rotundigenitalis Yű, 1933 was found on the inner operculum of M. cephalus. This species is characterized by a subcircular cephalothorax shield, 2-segmented abdomen with smaller proximal segment, and a 3-segmented antenna. This caligid copepod was also identified after Ho and Lin (2004).
Caligus sp. was collected from the gill filaments of J. coitor. The specimens are characterized by a subcircular cephalothorax shield, the presence of a sterna furca, small abdomen, and a well-developed fourth leg. This species could not be identified to the species level and might represent a currently undescribed species. Parapetalus hirsutus (Bassett-Smith, 1898) was found on the inner operculum of E. tetradactylum. It is characterized by a subcircular cephalothorax shield, 1-segmented large abdomen, oval caudal rami, and a 3-segmented antenna (Ho and Lin 2004).
Pseudocaligus sp. was obtained on the skin of S. argus. The characters of Pseudocaligus are similar to those of Caligus, except for a vestigial fourth leg or the complete absence of this leg (Ho and Lin 2004). This species is characterized by a round cephalothorax, short abdomen, and the presence of a sternal furca. The morphology of the present specimens was identical with the diagnosis of Pseudocaligus given by Ho and Lin (2004).
P. hirsutus was collected from the inner operculum of E. tetradactylum. The shape of this species is different with Caligus and it is characterized by a subcircular cephalothorax shield, 1-segmented large abdomen, caudal rami oval, and a 3-segmented antenna.
Family: Lernaeopodidae Milne Edwards, 1840
Two species of the family Lernaeopodidae were isolated. Naobranchia cf. polynemi Tripathi, 1962 occurred on the gill filament of E. tetradactylum (Fig. 4h). In the present study, only a single nongravid female was found, and was identified by using the original species description by Tripathi (1962). However, the description is brief and N. polynemi has not been redescribed. Thysanote sp. was isolated from the nasal cavity of S. argus. It is characterized by the short cephalothorax, a large trunk, and unbranched processes at the hind end of the trunk. The species was identified to the genus level by using the key of lernaeopodid genera by Boxhall and Halsey (2004).
Family: Lernanthropidae Kabata, 1979
Two species of lernanthropid copepods were found. Lernanthropus polynemi Richiardi, 1881 was found on the gill filaments of E. tetradactylum. This species can be identified by its cephalothorax shape, the subtriangular head, the shape of the urosome, and the straight egg sacs. The morphological features of the specimen correspond to the redescription by Piasecki and Hayward (2002). Another species of the Lernanthropidae, Lernanthropus sp., was collected from the gill filaments of J. coitor. According to Boxshall and Halsey (2004), the genus Lernanthropus consists of 119 species, but an identification key is not available.
Family: Pennellidae Burmeister, 1835
One adult and one larval stage of the family Pennellidae were found. Peniculus cf. scomberi Gnanamuthu, 1951 was collected from the dorsal fin of C. sexfasciatus and J. coitor (Fig. 4f). This species is characterized by an oval, short cephalothorax with a prominent proboscis-like mouth cone. Two narrow free thoracic segments are interposed between the cephalothorax and the genital complex, forming a neck-like structure. The body shape corresponds to the original description of P. scomberi by Gnanamuthu (1951). However, the original description is insufficient (Alexander 1983) and needs redescription. The larval pennellids attached on the gill filaments and gill rackers of E. coioides could not be further identified (Fig. 4g). They are characterized by attachment organs (frontal filament), the presence of a mouth cone and curious structures around the mouth cone. These characters allow assignment to the family Pennellidae; however, the life cycle and developmental stages of this family are widely unknown.
Class: Malacostraca Latreille, 1806
Order: Isopoda Latreille, 1817
Family: Cymothoidae Leach, 1818
One adult Isopoda, Cymothoa sp., was isolated from the mouth cavity of S. argus. The genus Cymothoa is mainly characterized by the general body shape (cephalon sunken into preonite), and the morphology of the pleon (distinct from pereon) and the cephalon. The actual species composition of the genus Cymothoa is unclear and a revision is needed (Bunkley-Williams and Williams 1998).
Family: Gnathiidae Harger, 1880
The pranzia stages of gnathiid isopods were found on the gill filaments of S. argus and C. sexfasciatus. The pranzia stages could be identified to the family level only due to the lack of species-specific characters. Gnathiid larvae can be recognized by its characteristic body shape, resembling a thin female with large eyes. The living larvae are characterized by a red color due to the uptake of large quantities of blood.
Discussion
According to Froese et al. (1996) and Tomascik et al. (1997), Indonesia is part of the world’s center of marine aquatic biodiversity. The exceptionally high biodiversity of the marine fauna in the Indonesian Archipelago is a result of its geographical location and geological history. Though less than 10% of the Indonesian marine and brackish water fish fauna have yet been studied, the parasite fauna appears to be highly diverse. Parasites are an important but often underestimated part of marine biodiversity (Marcogliese and Price 1997). Palm et al. (1999) estimated that more than three different metazoan parasites occur in each marine fish species, leading to the assumption that more than 9,000 metazoan marine fish parasites occur in Indonesia. To date, 242 marine and brackish water fish species have been studied for their parasite fauna in Indonesian waters, revealing more than 400 species. However, the Indonesian fish species are far from being fully studied and in some regions, nobody has ever worked on marine fish parasites.
This is the first study on fish ectoparasites from Segara Anakan Lagoon. A species-rich ectoparasite fauna was recorded. Twenty-two species or genera were recorded for the first time from Indonesian waters or from the southern coast of Java. In addition, eight new host records could be established. The collected crustaceans consist of typical shallow water species. According to Boxshall and Halsey (2004), caligid, ergasilid, and lernanthropid copepods are known as common parasites of shallow water fish. Copepods of the family Ergasilidae are mostly known as freshwater parasites, and only few species are known from the brackish water or marine environment (Boxshall and Halsey 2004). The other collected copepod families (Bomolochidae, Caligidae, Lernanthropidae, Lernaeopodidae, and Pennellidae) are mainly or exclusively known as marine fish parasites (Kabata 1979; Boxshall and Halsey 2004), and also the isopods Cymothoa sp. and gnathiids are known as ectoparasites of marine fish (Möller and Anders 1986).
Within the present study, M. cephalus, S. argus, E. tetradactylum, and J. coitor had a species-rich copepod fauna. Six parasitic copepods were recorded from M. cephalus. Even though this fish species has a wide distribution and has been well studied for copepod parasites (e.g., Paperna and Overstreet 1981; El-Rashidy and Boxshall 1999), several copepods from the present study represent new host records. According to El-Rashidy and Boxshall (1999), at least nine different ergasilid genera have been recorded from mugilid fish, and M. cephalus alone harbors six different genera (Dermoergasilus, Ergasilus, Mugilicola, Nipergasilus, Paraergasilus, and Thersitina). Three species of ergasilid copepods were recorded from Segara Anakan; two of them belonging to Ergasilus and one species probably represents an undescribed genus. S. argus was infested with seven copepod species, and showed the highest number of species. Beside copepods, the parasitic isopod Cymothoa sp. was found to infest S. argus. Isopods belonging to Cymothoa are common in the mouth cavity of their hosts (Bunkley-Williams and Williams 1998). Mladineo and Valic (2002) observed the mechanism of infection in cymothoid isopods. The larvae actively search their fish hosts after leaving the female, and S. argus is a suitable host for Cymothoa sp.
In contrast to the fish species mentioned above, E. coioides, J. coitor, S. javus, and C. sexfasciatus harbored only a low number of host specific copepods. According to Boxshall and Halsey (2004), most parasitic copepods are known to have high host specificity. For example, L. polynemi and P. hirsutus occur exclusively on polynemid fish (Pillai 1962; Ho and Lin 2001; Piasecki and Hayward 2002). Others such as some Caligus spp. are known to have low hosts specificity (Ho and Lin 2004). For example, Caligus elongatus Nordmann, 1832 has been recorded from more than 100 host species, both teleosts and even elasmobranchs, belonging to 47 families (Williams and Bunkley-Williams 1996). Within the present study, seven out of eight fish species were infested with Caligus spp. Similarly, the gnathiid isopods on S. argus and C. sexfasciatus show low host specificity. According to Möller and Anders (1986), pranzia stages were recorded to infest a high number of different fish species. Most copepods from Segara Anakan were host-specific, with 19 species infesting only a single host fish species. However, some species such as C. epidemicus and C. rotundigenitalis are known to infest several fish species, thus having a low level of host specificity such as the isolated isopods.
Segara Anakan is a brackish water environment and seems to exclude some typical marine helminths such as the trypanorhynch cestodes (Yuniar 2005). Remane (1934) developed a diagram to describe the faunistic distribution along a marine freshwater salinity gradient. According to him, the true brackish water species are less diverse than those living in marine or freshwater environments. According to Nybakken (2001), three faunistic components occur within estuaries, marine, freshwater, and brackish water or estuarine species. The species composition depends on the actual salinity. Beside true brackish water species, additionally, some freshwater and marine species with a high salinity tolerance can be found in such environments (Nybakken 2001). Zander (1998) published a review on parasites in brackish water environments based on research in the Baltic Sea, the largest brackish water environment in Europe. The author stated that the parasite fauna in brackish water regions is poor relative to truly marine habitats, host specificity is low, new hosts have been acquired, the number of hosts in life cycles is reduced, and there are adaptations to brackish water hosts. Studies on fish parasites in brackish water habitats in tropical areas are scarce and have been mainly carried out on copepods in India and Brazil (Babu and Raj 1985; Boxshall and Montú 1997). Data from Indonesian waters are lacking. Within the present study, the parasites collected from Segara Anakan Lagoon show a wide salinity range. According to Zander (1997), within a salinity of 6–8 ppt, the species diversity is minimal. Twenty-four parasitic crustacean species were recorded in the studied brackish water Segara Anakan, showing high ectoparasite richness. This might be explained by a still high salinity ranging from 19–31 ppt within the lagoon, close to the high salinity in marine environments. This contrasts, however, the observation that some marine helminth species do not enter the lagoon, though being common in the marine system along the southern Java coast (Yuniar 2005, Palm 2004, Jakob and Palm 2006).
Importance of fish parasites for future mariculture development in Segara Anakan Lagoon
Marine finfish is often cultivated in coastal fish ponds. The floating net cage-culture system is mainly extensive and expanding rapidly throughout Southeast Asia. These systems nowadays serve as an important source of fish for the local and international markets (Leong 1992). The impact of parasites on marine finfish culture is well documented, with catastrophic losses reported for disease outbreaks that result in high mortality levels. Prerequisite for establishing effective control measures in fish cultures is a quick and exact diagnosis of the causative agent, and the knowledge on the complex host–parasite–environment relationships (Moravec 1994). The correct parasite identification is of great significance, and may decide on the survival or death of the infested fish. Today, more than 60 parasite species are known that directly affect the Indonesian mariculture. Unfortunately, identification of many of these parasites is still unclear.
According to Ho (2000) and Johnson et al. (2004), caligid copepods are economically important parasites in mariculture facilities. Within Segara Anakan, 10 species of caligid copepods were collected from eight fish species. C. acanthopagri, C. epidemicus, and C. rotundigenitalis already caused severe problems within the Asian mariculture (Ho and Lin 2004). In Indonesia, several cases of fish mortalities related to the infestation with Caligus spp. have been recorded, such as in grouper culture in Gondol Research Station, Bali (Yuasa et al. 1998; Zafran et al. 2000). Lin and Ho (1998) stated that two species belonging to the family Ergasilidae were found on fish cultured in brackish water in Taiwan. Within the present study, five species of ergasilid copepods were recorded from Segara Anakan Lagoon. Lernanthropus sp. was recorded as a parasite of snapper (L. johnii) cultured in floating net cages in Malaysia (Leong and Wong 1989). Lernanthropid copepods were also collected in Segara Anakan, infesting J. coitor and E. tetradactylum.
Isopods are also common parasites in mariculture facilities. Papapanagiotou and Trilles (2001) listed several isopods of the family Cymothoidae, which are known to infest cultivated marine fish, also causing great financial loss. For example, Ceratothoa parallela (Otto, 1828) caused over 50% mortality of Sparus aurata L., 1758 cultured in Greece (Papapanagiotou and Trilles 2001). Isopods on fish cultured along the Peruvian coast were published by Williams and Bunkley-Williams (2000). The authors recorded that Ceratothoa gaudichaudii (Milne Edwards, 1840) caused a 15% loss in body weight, costing approximately 1.3 billion kilograms of annual loss to the fishermen. Cymothoa oestrum (L., 1793) was reported to cause superinfections in caged fishes in the Caribbean (Williams and Bunkley-Williams 2000). In Indonesia, problems in mariculture facilities in some localities were caused by Isopoda. For example cymothoid isopods were recorded in the nasal and gill cavity of groupers cultured in Bali (Koesharyani et al. 2001). In Segara Anakan Lagoon, one species of Isopoda of the same family was found in the mouth cavity of S. argus. This species might also become a problem in future mariculture activities within the region.
Conclusions
The present study of ectoparasites from commercial fish species in Segara Anakan Lagoon demonstrates a high parasite richness within this tropical brackish water environment. All recorded species are typical shallow water species, and some of them are known as economically important pathogens within the Asian mariculture. Consequently, the brackish water environment does not prevent disease outbreaks due to parasitic copepods by preventing pathogenic marine or freshwater species to enter the lagoon. This might cause disease problems if the Segara Anakan Lagoon would be developed for commercial finfish mariculture in the future. The studied fish species varied in the number of copepod species. These differences depend on the host specificity of the parasites and the habitat preference of the host. Most of the isolated copepods appear to have high host specificity within the lagoon, occurring on a single host fish species. However, with C. epidemicus and C. rotundigenitalis, also species with lower host specificity and wider host range were found. Salinity is considered one of the main factors influencing the parasite infestation with ectoparasitic copepods. Some euryhalin metazoan ectoparasites can tolerate a wider salinity range (e.g., ergasilid copepods), but most species found are stenohalin and prefer the marine environment. The parasitic copepod fauna of Segara Anakan Lagoon is dominated by marine species, all of them with obviously a high degree of salinity tolerance, thus being euryhalin. The present study demonstrates a high ectoparasitic crustacean richness also in a brackish water tropical ecosystem. This and further studies will determine the actual contribution of the Indonesian parasite fauna to marine biodiversity. Larger samples and studies from other Indonesian regions are needed to describe and analyze the real parasite diversity and to verify the potential threats of fish parasites for the Indonesian mariculture industry.
References
Alexander PD (1983) Peniculus haemuloni, a new species of copepod (Siphonostomatoida: Pennellidae) parasitic on Haemulon steindachneri from Ubatuba, Brazil. Bull Br Mus Nat Hist Zool 45:381–385
Babu SJ, Raj PJS (1985) Copepod parasites of fishes of Pulicat Lake. Proc Symp Coast Aquacult 3:941–950
Boxshall GA, Halsey SH (2004) An introduction to copepod diversity, vol 1 and 2. The Ray Society, London
Boxshall GA, Montú MA (1997) Copepods parasitic on Brazilian coastal fishes: a handbook. Nauplius 5:1–225
Bunkley-Williams L, Williams EH Jr (1998) Isopods associated with fishes: a synopsis and corrections. J Parasitol 84:893–896
Bush AO, Lafferty KD, Lotz JM, Shostak AW (1997) Parasitology meets ecology on its own terms: Margolis et al. revisited. J Parasitol 83:575–583
Dewi J, Purnomowati R, Perdana DA (2003) Penanganan penyakit ikan. In: Penanganan penyakit ikan budidaya laut. Balai Budidaya Laut Direktorat Jenderal perikanan budidaya Departemen Kelautan dan Perikanan. Lampung:14–21
Dudley RG (2000) Segara Anakan fisheries management plan. Segara Anakan conservation and development project, components B & C. Consultant’s report 33 pp
El-Rashidy H, Boxshall GA (1999) Ergasilid copepods (Poecilostomatoida) from the gills of primitive Mugilidae (grey mullets). Syst Parasitol 42:161–186
Froese R, Luna SM, Capuli EC (1996) Checklist of marine fishes of Indonesia, compiled from published literature. In: Pauly D, Martosubroto P (eds) Baseline studies of biodiversity: the fish resources of Western Indonesia. ICLARM Stud Rev 23:217–275
Gnanamuthu CP (1951) Three species of lernaeid copepods parasitic on South Indian fish. Ann Mag Nat Hist 4:77–86
Harris E (2000) Studies of Indonesian fisheries today and the research needed. In: Carman O, Sulistiono, Purbayanto A, Suzuki T, Watanabe S, Arimoto T (eds) Sustainable fisheries in Asia in the new millennium. Proceedings of the JSPS-DGHE International Symposium on Fisheries Science in Tropical Area, pp 62–66
Hartono P, Dewi J, Tusihadi T (2001) Penyakit pada budidaya ikan kerapu. In: Pembesaran kerapu macan (Epinephelus fuscogutattus) dan kerapu tikus (Cromileptes altivelis) di karamba jaring apung. Departemen Kelautan dan Perikanan Direktorat Jenderal Perikanan Budidaya Balai Budidaya Laut Lampung :46–54
Ho J-S (2000) The major problem of cage aquaculture in Asia relating to sea lice. In: Liao IC, Lin CK (eds) Cage aquaculture in Asia. Proceedings of the first international symposium on cage aquaculture in Asia. Asian Fisheries Society, Manila, Philippines, and World Aquaculture Society—Southeast Asian Chapter, Bangkok, Thailand, pp 13–19
Ho J-S, Lin C-L (2001) Parapetalus occidentalis Wilson (Copepoda, Caligidae) parasitic on both wild and farmed cobia (Rachycentron canadum) in Taiwan. J Fish Soc Taiwan 28:305–316
Ho J-S, Lin C-L (2004) Sea lice of Taiwan (Copepoda: Siphonostomatoida: Caligidae). The Sueichan Press, Taiwan
Hutomo M (1986) Catatan tentang arti penelitian parasit dan parasitisme pada biota laut. Pewarta Oseana 6:5–7
Jakob E, Palm HW (2006) Fish parasites of commercial fish species from the southern Java coast, Indonesia, including the distribution pattern of trypanorhynch cestodes. Verhandlungen der Gessellschaft lür lchthyologie 5:165–191
Johnson SC, Treasurer JW, Bravo S, Nagasawa K, Kabata Z (2004) A review of the impact of parasitic copepods on marine aquaculture. Zool Stud 43:229–243
Kabata Z (1979) Parasitic Copepoda of British fishes. The Ray Society, London
Kabata Z (1985) Parasites and deseases of fish cultured in the tropics. Taylor and Francis, London and Philadelphia, 318 pp
Koesharyani I, Roza D, Mahardika K, Johnny F, Zafran, Yuasa K (2001) Manual for fish disease diagnosis-II. Marine fish and crustacean diseases in Indonesia. Gondol Research Institute for Mariculture. Central Research Institute for Sea Exploration and Fisheries, Department of Marine Affairs and Fisheries and Japan International Cooperation Agency, 49 pp
Leong TS (1992) Diseases of brackish water and marine fish cultured in some Asian countries. In: Shariff M, Subasinghe RP, Arthur JR (eds) Diseases in Asian aquaculture I. Proceedings of the first Symposium on Diseases in Asian Aquaculture, 26–29 November, 1990, Bali, Indonesia. Fish health section, Asian Fisheries Society, Manila, Philippines, pp 223–236
Leong TS, Wong S-Y (1989) Parasites of wild and cultured golden snapper, Lutjanus johnii (Bloch), in Malaysia. Trop Biomed 6:73–76
Lin C-L, Ho J-S, Shen S-N (1994) Two species of Cligus (Copepoda: Caligidae) parasitic on black sea bream (Acanthopagrus schlegeli) cultured in Taiwan. Fish Patrol 29:253–264
Lin C-L, Ho J-S (1998) Two new species of ergasilid copepods parasitic on fishes cultured in brackish water in Taiwan. Proc Biol Soc Wash 111:15–27
Marcogliese DJ, Price J (1997) The paradox of parasites. Glob Biodivers 7:7–15
Mladineo I, Valic D (2002) The mechanisms of infections of the buccal isopod Ceratothoa oestroides (Risso, 1836), under experimental conditions. Bull Eur Assoc Fish Pathol 22:304–310
Möller H, Anders K (1986) Diseases and parasites of marine fishes. Verlag Möller, Kiel
Moravec F (1994) Parasitic nematodes of freshwater fishes of Europe. Academia, Praha
Naamin N (1991) The ecological and economic roles of Segara Anakan, Indonesia, as a nursery ground of shrimp. In: Chou LM, Chua TE, Khoo HW, Lim PE, Paw JN, Silvestre GT, Valencia MJ, White AT, Wong PK (eds) Towards an integrated management of tropical coastal resources. ICLARM Conf Proc 22:119–130
Nybakken JW (2001) Marine biology: an ecological approach. Benjamin Cummings, an imprint of Addison Wesley Longman, Incorporation, USA, p 516
Palm HW (2000) Trypanorhynch cestodes from Indonesian coastal waters (East Indian Ocean). Folia Parasitol 47:123–134
Palm HW (2004) The Trypanorhyncha Diesing, 1863. PKSPL-IPB Press, Bogor
Palm HW, Klimpel S, Bucher C (1999) Checklist of metazoan fish parasites of German coastal waters. Ber Inst Meereskd Christian-Albrecht-Univ Kiel 307:148
Papapanagiotou EP, Trilles JP (2001) Cymothoid parasite Ceratothoa parallela inflicts great losses on cultured gilthead sea bream Sparus aurata in Greece. Dis Aquat Org 45:237–239
Paperna I, Overstreet RM (1981) Parasites and diseases of mullets (Mugilidae). Aquaculture of grey mullets. In: Oren OH (ed) International Biological Programm 26. Cambridge University Press, USA, pp 411–493
Piasecki W, Hayward CJ (2002) Redescription of the fish parasite Lernanthropus polynemi Richiardi, 1881 (Copepoda: Siphonostomatoida) and relegation of two congeners to synonymy. Syst Parasitol 52:137–144
Pillai NK (1961) Copepods parasitic on south Indian fishes. Part I. Caligidae. Bull Centr Res Inst Univ Kerala, Ser C 8:87–130
Pillai NK (1962) A revision of the genera Parapetalus Steenstrup and Lütken and Pseudopetalus nov. Crustaceana 3:285–303
Pollunin NVC (1983) The marine resources of Indonesia. Oceanography and Marine Biology: An Annual Review 21:455–531
Pritchard MH, Kruse OW (1982) The collection and preservation of animal parasites. University of Nebraska Press, 318 pp
Remane A (1934) Die Brackwasserfauna. Zool Anz (Suppl) 7:34–74
Robinson DG, Ehlers U, Herken R, Hermann B, Mayer F, Schürmann FW (1985) Präparationsmethodik in der Elektronenmikroskopie. Springer-Verlag, Berlin, 208 pp
Romimohtarto K, Hutagalung H, Razak H (1991) Water quality of Segara Anakan-Cilacap (Central Java, Indonesia) with a note on lagoon fishery. In: Chou LM, Chua TE, Khoo HW, Lim PE, Paw JN, Silvestre GT, Valencia MJ, White AT, Wong PK (eds) Towards an integrated management of tropical coastal resources. ICLARM Conf Proc 22:131–141
Roza D, Johnny F, Kawahara S, Hanafi A (2002) Penyakit pada budidaya ikan kerapu dan upaya penanggulangannya. Kumpulan Makalah Seminar Pengembangan Teknologi Budidaya Kerapu, Balai Budidaya Laut Lampung, 2 Juli 2002:75–86
Sugama K (1999) Inventariasi dan identifikasi teknologi budi daya laut dan pantai yang telah dikuasai untuk diseminasi. In: Seminar nasional penelitian dan diseminasi teknologi budi daya laut dan pantai. Jakarta, December 2nd 1999:61–72
Tomascik T, Mah AJ, Nontji A, Moosa MK (1997) The Ecology of the Indonesian Seas. Part II. Periplus Editions (HK) Ltd., Singapore
Tripathi YR (1962) Parasitic copepopods from Indian fisher. III Family Anthosomatidae and Dishelestiidae. Proceedings of the first All-Indian Congress of Zoology 2:191–217
White AT, Martosubroto P, Sadorra MSM (1989) The coastal environment profile of Segara Anakan-Cilacap, South Java, Indonesia. ICLARM. Association of Southeast Asian Nations. United States Coastal Resources Management Project. Techn Publ Ser 4:81
Williams EH Jr, Bunkley-Williams L (1996) Parasites of offshore big game fishes of Puerto Rico and the western Atlantic. Antillean College Press, Mayaguez
Williams EH Jr, Bunkley-Williams L (2000) Multicellular parasite (macroparasite) problems in aquaculture. In: Stickney RR (ed) Encyclopedia of Aquaculture. Wiley, USA
Yuasa K, Zafran, Koesharyani, Rosa D, Jonny F (1998) Diseases in marine fishes reared at Gondol Research Station for coastal fisheries. Prosid Semin Teknol Perikanan Pantai:94–98 (Bali 6–7 August 1998)
Yuniar AT (2005) Parasites of marine fish from Segara Anakan, Java, and its possible use as biological indicators. MSc Thesis, University of Bremen, Germany, p 90
Zafran, Roza D, Johnny F, Koesharyani I, Yuasa K (2000) Diagnosis and treatments for parasitic diseases, humpback grouper, Cromileptes altivelis broodstock. Gondol Research Station for Coastal Fisheries, Central Research Institute for Fisheries, Indonesia, p 8
Zander CD (1997) Parasit-Wirt-Beziehungen: Einführung in die ökologische Parasitologie. Springer, Berlin Heidelberg New York
Zander CD (1998) Ecology of host parasite relationships in the Baltic Sea. Naturwissenschaften 85:426–436
Acknowledgements
We would like to thank Prof. H. Mehlhorn (Institut für Zoomophologie, Zellbiologie und Parasitologie, Heinrich-Heine-Universität Düsseldorf) for the possibility to carry out parts of scanning electron microscopy. We would also like to thank Prof. G.A. Boxshall (National Museum of Natural History, London) and Prof. J.-S. Ho (Department of Biological Sciences, California State University, California) for confirmation of some copepod identification and literature support. We also thank SPICE project members Dr. T. Jennerjahn (ZMT Bremen), E. Ardli, M.Sc. (Jenderal Soedirman University and ZMT Bremen), and Dr. B. Heru, Arthadi, M.S. and S. Subadrah, S.U. of the Parasitology and Entomology Laboratory, Jenderal Soedirman University (UNSOED), Purwokerto, for their support during data collection. Financial support was provided by the German Academic Exchange Service (DAAD), the German Federal Ministry of Education and Research (BMBF), and the German Research Council PA 664/4-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuniar, A.T., Palm, H.W. & Walter, T. Crustacean fish parasites from Segara Anakan Lagoon, Java, Indonesia. Parasitol Res 100, 1193–1204 (2007). https://doi.org/10.1007/s00436-006-0391-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0391-9