Abstract
Neospora caninum is a tissue cyst-forming coccidium that may cause neuromuscular disorders in dogs. Infected bitches can transmit the parasite to their pups in utero. Vertical transmission may occur after primary infection during pregnancy and in subsequent pregnancies. The reason why only a few pups develop clinical neosporosis is unknown. We obtained sera from a Doberman bitch and its offspring delivered in three litters. The bitch had a titer of 1:640 in an indirect fluorescent antibody test (IFAT). At least three pups of litter A, one pup of litter B, and two pups of litter C were also seropositive for N. caninum. However, clinical neosporosis developed only in one pup of litter C, which had the highest IFAT titer (1:5,120) of all dogs examined. Western blots carried out after one-dimensional and two-dimensional separation of N. caninum tachyzoites revealed that the largest number of antigens was recognized by sera derived from the bitch. The lowest number of antigens was recognized by serum from the pup with clinical neosporosis. However, this pup uniquely recognized a major antigen with a molecular weight of about 17,000. The information collected in this study adds to our knowledge on why some pups develop clinical neosporosis and others do not.
Similar content being viewed by others
Introduction
In 1984, a neuromuscular disease was described for dogs in Norway, the causative agent of which was found to be a protozoal parasite that was morphologically but not serologically similar to Toxoplasma gondii (Bjerkås et al. 1984). Four years later, this parasite was also detected in dogs in the USA and was given the name Neospora caninum (Dubey et al. 1988a). The development of an immunohistochemical test specific for N. caninum enabled retrospective studies on fixed tissues which showed that this parasite was identical to the parasite found in the Norwegian dogs (Bjerkås and Dubey 1991) and that infections with N. caninum had previously also been present in Australia, New Zealand, South Africa, and Germany but were often misdiagnosed as infections with T. gondii (Dubey et al. 1990; Munday et al. 1990; Jardine and Dubey 1992; Burkhardt et al. 1992; Patitucci et al. 1997). Since then, canine infections with N. caninum have been diagnosed in many countries of the world (Dubey and Lindsay 1996; Lindsay and Dubey 2000).
N. caninum is heteroxenous and, in addition to dogs, can use several mammalian species as intermediate hosts (Dubey and Lindsay 1996; Lindsay and Dubey 2000). There is serological evidence that other canines, such as dingos, coyotes, and foxes, may also be infected with N. caninum (Almería et al. 2002; Barber et al. 1997; Buxton et al. 1997; Lindsay et al. 1996; Schares et al. 2001a). Thus far, dogs and coyotes have also been found to shed oocysts of N. caninum, and hence these species may serve as both intermediate and definitive hosts for the parasite (McAllister et al. 1998; Basso et al. 2001; Gondim et al. 2004).
It is believed that only intermediate hosts of N. caninum develop clinical neosporosis. In dogs, N. caninum may be transmitted vertically from the bitch to its offspring (Dubey and Lindsay 1996; Lindsay and Dubey 2000). In most cases, bitches are unaffected by the parasite and appear clinically normal. However, once infected, bitches can transmit the parasite in several consecutive pregnancies (Dubey et al. 1990; Dubey and Lindsay 1996). To date, it is not clear under which circumstances pups that are born to these bitches develop clinical neosporosis.
In this study, we describe a study on antibody reactions in a Doberman family in which the bitch and at least two-thirds of its offspring of three consecutive litters showed serological evidence of infection with N. caninum. One pup of litter C suffered from clinical neosporosis, but the bitch and all other offspring remained asymptomatic for the length of the observation period which was up to 9 1/4 years.
Materials and methods
Parasites
Tachyzoites of the N. caninum Nc-1 strain were grown in Vero cells at 37°C and 5% v/v CO2 using Iscoves’ Modified Dulbecco's Medium supplemented with 200 U/ml penicillin, 200 μg/ml streptomycin, and 2–5% v/v horse serum. When most of the infected Vero cells were ruptured, tachyzoites were harvested by scraping the parasites and the remaining host cells into the growth medium followed by centrifugation at 1,000 × g for 10 min. The pellet was suspended in 5–10 ml phosphate-buffered saline (PBS, pH 7.4) and forced through a 27-gauge needle to disrupt any remaining host cells. The suspension was then washed twice in PBS, and the parasites were purified from host-cell debris by filtration through a 5-μm hydrophilic polyvinylidene difluoride membrane (Durapore, Millex-SV, Millipore). Purified tachyzoites were concentrated by centrifugation, and dry pellets of tachyzoites were stored in liquid nitrogen until use.
Dog sera
Serum samples were collected from the Doberman pup of litter C with clinical neosporosis at the time of euthanasia and retrospectively from the bitch and those dogs of its offspring whose owners agreed that their dogs could participate in the study. In total, four of nine dogs from litter A, two of five dogs from litter B, and six of eight dogs from litter C were included in the study (Fig. 1). In addition, follow-up sera were taken from the bitch and two dogs of litter C (Table 1). Serum samples were also collected from an unrelated male Doberman that lived in the same household as the Doberman bitch. All serum samples were stored at −20°C until use. Serum derived from an unrelated dog that did not have contact to the Doberman family was used as negative control serum.
Family diagram of a Doberman bitch and its three consecutive litters. The bitch delivered the litters at an age of 3.5, 5.5, and 6.5 years. The pups are numbered consecutively (A1–C22). The sex of the pups is indicated as: m male, f female, or ? unknown. Pup C15 (in square) had a confirmed clinical neosporosis, whereas neither the bitch nor the siblings showed any signs of the disease
Indirect fluorescent antibody test (IFAT)
Tachyzoites of the N. caninum Nc-1 strain were harvested from cell culture as described above, rinsed in PBS, and adjusted to a concentration of 1 × 106/ml. IFAT microscope slides were prepared by dropping 10 μl of that suspension into each well, air-dried, and stored at −80°C until use. Before each test, IFAT slides were removed from storage, thawed at 22°C, fixed for 10 min in methanol, and then washed for 10 min in PBS on a magnetic stirrer with slight movement. Sera were diluted in PBS in twofold steps starting at a dilution of 1:20, and 10 μl of each serum dilution was dispensed into separate wells of the IFAT slides. The slides were then incubated in a moist chamber at 37°C for 30 min, rinsed with PBS, and washed as described above. The secondary antibody was a rabbit anti-dog IgG (H + L) conjugated to fluorescein-isothiocyanate (Jackson ImmunoResearch), which was used at a dilution of 1:64 in PBS containing 0.25% w/v Evan's blue. Ten microliters of this dilution was dispensed into each well, and the slides were incubated and washed as before. They were then air-dried, overlaid with glycerine containing 10% v/v PBS, and covered with a cover slip. The microscope slides were examined using a florescence microscope (wavelength 450–490 nm, 400× magnification), and the degree of fluorescence was scored as 3+, 2+, 1+, trace, and negative. The highest serum dilution showing a 1+ fluorescence was taken as the IFAT titer.
One-dimensional polyacrylamide gel electrophoresis (PAGE) and Western blotting
Pellets containing 1 × 107 tachyzoites of the N. caninum Nc-1 strain were suspended in 160 μl of 2× Laemmli buffer containing 200 mM dithiothreitol (DTT) and boiled at 98°C for 7 min. Forty-microliter samples were loaded onto a polyacrylamide gel (PAG, stacking gel: 3.5%, running gel: 12.5%, 30% acrylamide/bis-acrylamide solution, Sigma), and electrophoresis was carried out under denaturing conditions in a Mini-PROTEAN® 3 cell (BioRad) for 1.5 h (constant voltage of 100 V for the stacking gel and 150 V for the running gel) in sodium dodecyl sulfate (SDS) PAGE buffer (0.2 M glycine, 0.025 M Tris, 0.1% w/v SDS). Protein molecular weight (mol. wt.) markers ranging from 6,500 to 175,000 (Prestained Protein Marker, Broad range, BioRad) were included on all gels.
Proteins were transferred to a nitrocellulose membrane with a pore size of 0.45 μm (Protran®, Schleicher and Schuell) using a semidry electrophoretic blotting unit (Trans-Blot® SD, BioRad). Blotting was carried out at 22°C and 15 V for 30 min using transfer buffer (20 mM Tris, 150 mM glycine, 10% v/v methanol). The membrane was cut into strips which were processed separately. Membrane strips containing the marker were stained with Ponceau S, and strips containing the antigen were processed as follows. Free binding sites were blocked with 5% w/v powdered skim milk in PBS at 22°C for 15 min. The strips were incubated with dog sera diluted 1:100 in PBS containing 5% w/v powdered skim milk at 4°C overnight and then washed four times at 22°C for 10 min in PBS containing 0.1% v/v Tween 20 and once for 2 min with PBS only. Membrane strips were incubated with rabbit anti-dog IgG (H + L) conjugated to horse radish peroxidase (Jackson ImmunoResearch) diluted 1:20,000 in PBS containing 5% w/v powdered skim milk at 22°C for 45 min. After washing as described above, antigenic polypeptide bands were developed by chemoluminescence (ECL Plus Western Blotting Detection Reagents, Amersham Biosciences).
Two-dimensional PAGE and Western blotting
For two-dimensional (2D) PAGE, total protein extracts were prepared by dissolving tachyzoites of the N. caninum Nc-1 strain in lysis buffer containing 9.25 M urea, 2% v/v Triton X-100, 2% v/v mercaptoethanol, and 2% v/v Pharmalyte carrier ampholytes (Pharmalyte, range pH 4–7, Amersham Pharmacia Biotech). The protein concentrations of the lysates were estimated by a Bradford dye-binding assay using a γ-globulin standard, and the protein concentration of each lysate was adjusted to 1 mg/ml (corresponding to 4×106 tachyzoites) of total protein in lysis buffer.
2D PAGE was carried out on a horizontal Multiphor II Electrophoresis Unit (Amersham Pharmacia Biotech). One-dimensional (1D) isoelectric focusing was carried out on immobilized pH gradients (IPG) gels covering a medium pH range of 4–7 (Immobiline DryStrip pH 4–7, Amersham Pharmacia Biotech). The IPG gel strips were rehydrated for 6 h at 22°C in a rehydration solution containing 8.5 M urea, 0.5% v/v Triton X-100, 0.1 M DTT, 0.05 mM acetic acid, and a trace of Orange G. After rehydration, 100-μl samples corresponding to 0.2 mg total tachyzoite protein extract were loaded onto the IGP gels from both the anodic and cathodic ends of the strip using sample cups (Amersham Pharmacia Biotech). Gels were prefocused at 500 V for 1 h followed by focusing at 1,400 V overnight at 20°C.
Ultrathin (0.5 mm) PAG with an exponential pore gradient (12–18% T, 2% C) and a gel size of 24 × 11 cm were cast on a plastic support (GelBond PAGfilm, Amersham Pharmacia Biotech) for 2D SDS-PAGE separations. Before their application to the 2D PAG, IPG gel strips were equilibrated in two steps, each for 15 min at 22°C. Equilibration buffer contained 25 mM Tris–HCl pH 8.8, 6 M urea, 30% v/v glycerol, 2% w/v SDS, and a trace of bromphenol blue to which 1% w/v DTT was added for the first step and 4.8% w/v iodoacetamide for the second step. The IPG gel strips were placed onto a 2D PAG, and electrophoresis was carried out under denaturing conditions at 15°C and a maximum voltage of 700 V (50 mA, 4 W limiting) for 6 h. Protein mol. wt. markers ranging from 14,400 to 187,000 were included on all 2D PAG (LMW Marker Kit, Amersham Pharmacia Biotech).
Tachyzoite protein maps were transferred from the 2D PAG to a supported nitrocellulose membrane with a 0.2-μm pore size (Optitran BA-S 83, Schleicher and Schuell) using a semidry electrophoretic blotting unit (Multiphor II NovaBlot Unit, Amersham Pharmacia Biotech). Blotting was carried out at 22°C and a constant 250 mA for 1 h using a discontinuous buffer system. The membrane was then washed three times for 10 min at 22°C in Tris-buffered saline (pH 8.0) containing 0.05% v/v Tween 20 (TBST). Free binding sites were blocked with 5% w/v bovine serum albumin (BSA, fraction V powder) in TBST at 4°C overnight. The membrane was washed as described above and probed for 1 h at 22°C with dog serum diluted 1:40 in TBST containing 2% w/v BSA. The membrane was washed as before and then incubated for 30 min at 22°C with rabbit anti-dog IgG (H+L) conjugated to alkaline phosphatase (Jackson ImmunoResearch) diluted 1:1,000 in TBST containing 2% w/v BSA. The membrane was washed as before, and antigenic protein spots were developed with AP buffer (0.1 M Tris–HCl, 0.1 M NaCl, 10 mM MgCl2, pH 9.5) containing 0.66% w/v nitro blue tetrazolium and 0.33% w/v 5-bromo-4-chloro-3-indolyl phosphate. Color reaction was stopped after 5 min by placing the membrane in deionized water.
Results
Clinical history
In the Doberman family examined in this study, the bitch gave birth to three litters when it was 3.5, 5.5, and 6.5 years old (Fig. 1). One pup of litter C (dog C15) was presented to a veterinary practice at the age of 8 weeks with coordination problems of its hind limbs, which worsened over the following week resulting first in an incomplete, then a complete paralysis of the hind limbs. The pup appeared dull and suffered from anorexia and urinary and fecal incontinence. It was vaccinated against parvovirosis, distemper, hepatitis, and leptospirosis, and no abnormality was observed hematologically. Despite treatment with vitamin B, penicillin, dexamethasone, and butylscopolamin, paralysis expanded to the front limbs at the age of 10 weeks. At that time, serological examination by IFAT revealed a titer of 1:5,120 to N. caninum and no antibodies to T. gondii. After induction of specific therapy with clindamycin and sulfadiazine/trimethoprim supplemented by vitamin B and dexamethasone, the pup's general condition and appetite returned to normal, and 10 days later, it could use its front limbs again. However, complete hind limb paralysis continued and resulted in severe muscle atrophy, so the pup was euthanized at the age of 16 weeks.
According to reports from the owners, neither the bitch nor any of its other offspring showed neuromuscular symptoms over the observation period, i.e., up to 9 1/4 years of age for the bitch, 3.5 years of age for litter A, 1.5 years of age for litter B, and 0.5 years of age for the siblings of dog C15. The unrelated male Doberman that lived in the same household as the Doberman bitch also was asymptomatic, at least up to an age of 9 years.
IFAT
The bitch and six of the 12 dogs of its offspring for which serum samples were available were positive in the IFAT (Table 1). The serum derived from the pup with clinical neosporosis (dog C15) had the highest antibody titer (1:5,120). By contrast, IFAT titers of the asymptomatic dogs were much lower, varying from 1:40 to 1:640. The unrelated male Doberman (dog 23) which was sampled at the same time as the bitch was also positive in the IFAT (1:320). There was little variation in IFAT titers (1:320 or 1:640) for the three serum samples of the bitch, which were taken at the age of 7, 7 1/2, and 9 1/4 years. The two serum samples of dog C20 taken at the age of 4 and 11 months were negative. Negative results were also obtained for the three serum samples of dog C19, which were taken at the age of 4 months, 11 months, and 7 years.
Western blots derived from 1D PAG
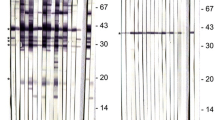
In the Western blots derived from 1D PAG, the sera of IFAT-positive asymptomatic dogs recognized five major antigenic polypeptides with mol. wt. of 17,000, 30,000, 33,000, 42,000, and 48,000 in different intensities (Fig. 2). All three sera of the bitch taken over a period of 2 1/4 years reacted strongly with the four high mol. wt. antigenic polypeptides (30,000 to 48,000) and moderately with the 17,000 mol. wt. polypeptide (Figs. 2 and 3a). The serum of dog A3 reacted strongly with the 30,000 and 33,000 mol. wt. polypeptides, moderately with the 17,000 mol. wt. polypeptide, and only weakly with the two other polypeptides. The sera of dogs B10 and C16 reacted strongly with the 30,000 or 33,000 mol. wt. polypeptides, while the other three polypeptides were recognized only weakly. The serum of the pup with congenital neosporosis (dog C15) recognized the 42,000 and 48,000 mol. wt. polypeptides but none of the three lower mol. wt. polypeptides.
Western blots derived from tachyzoite extracts of the N. caninum Nc-1 strain separated by 1D SDS-PAGE under denaturing conditions. Western blots were probed with serum collected from the bitch, IFAT-positive asymptomatic dogs (A3, B10, C16), IFAT negative asymptomatic dogs (B11, C18, C19, C20), the pup with clinical neosporosis (C15), or a control serum collected from an unrelated IFAT negative dog. Arrows (▶) mark five major antigenic polypeptides (mol. wt. 17,000, 30,000, 33,000, 42,000, and 48,000) that are detected by the sera of IFAT-positive asymptomatic dogs (bitch, A3, B10, and C16) in different intensities
Western blots derived from tachyzoite extracts of the N. caninum Nc-1 strain separated by 1D (a) or 2D PAGE (b) under denaturing conditions. Western blots were probed with sera of the bitch taken at the age of 7 and 9 1/4 years (a and b), sera of dog C19 taken at the age of 4 and 11 months (a and b), an unrelated male Doberman (dog 23), or a negative control serum. a Arrows (▶) mark five major antigenic polypeptides (mol. wt. 17,000, 30,000, 33,000, 42,000, and 48,000) that are detected by the sera of IFAT-positive asymptomatic dogs. b Dotted lines encircle antigenic spots that are detected by all sera of IFAT-positive dogs; striped lines encircle antigenic spots that are only detected by sera derived from IFAT-positive asymptomatic dogs
The antigen patterns recognized by the three sera of the bitch taken at different time points were highly reproducible (Fig. 3a) and similar to the antigen patterns recognized by the sera obtained from its IFAT-positive asymptomatic offspring and the unrelated male Doberman (Figs. 2 and 3a). The serum samples of the IFAT-negative dogs (C19 and C20) were also negative in the Western blot.
Western blots derived from 2D PAG
In the Western blots derived from 2D PAG, all sera from IFAT-positive asymptomatic dogs consistently detected three antigenic spots in the mol. wt. range of 11,000 to 18,000 and the pI range of 5–6, several antigenic spots in the high mol. wt. range of >60,000 and the pI range of 6–7, and a large number of antigenic spots in the intermediate mol. wt. range (20,000 to 30,000) and the pI range of 4.5–6.5 (Figs. 3b and 4). The recognition of antigenic spots by the sera of the bitch taken at different time points was highly reproducible (Fig. 3b).
Western blots derived from tachyzoite extracts of the N. caninum Nc-1 strain separated by 2D PAGE under denaturing conditions. Western blots were probed with serum collected from the bitch, sera from IFAT-positive asymptomatic dogs born in three litters (A1, A2, A3, B10, and C16), a pup with clinical neosporosis (C15) or its IFAT-negative siblings (C19 and C20). Dotted lines encircle antigenic spots that are detected by all sera of IFAT0-positive dogs; striped lines encircle antigenic spots that are only detected by sera derived from IFAT-positive asymptomatic dogs; the complete line encircles an antigenic spot that is only detected by the serum of the pup with clinical neosporosis
By contrast, no antigens in the high mol. wt. range (>60,000) and only few antigens in the medium mol. wt. range were recognized by the serum of pup C15 with clinical neosporosis (Fig. 4). Instead, this serum uniquely recognized one antigen with a mol. wt. of about 17,000 and a pI of about 4.5, which was not detected by any of the sera obtained from IFAT-positive asymptomatic dogs (Fig. 4).
Discussions
Most cases of clinical neosporosis in dogs have been observed in young dogs that were most likely infected congenitally (Rühlmann et al. 1995; Dubey and Lindsay 1996; Dubey 1999; Lindsay and Dubey 2000). Clinical signs of neosporosis are of the neuromuscular type, such as ataxia, ascending paralysis, paresis, muscle atrophy, and spastic hyperextension of the flexor tendons. In most cases, hind limbs are more affected than front limbs, but tetraplegia may also occur (Barber and Trees 1996; Dubey and Lindsay 1996).
Infected bitches may transmit the parasite in several pregnancies, but not all of their offspring may be affected. Infected pups are usually asymptomatic at birth, but clinical neosporosis may develop later in life (Flagstad et al. 1995; Van Ham et al. 1996; Barber and Trees 1998; Dubey et al. 1998; Reichel et al. 1998). In most cases described to date, pups of the first and subsequent litters were affected by clinical neosporosis (Cummings et al. 1988; Dubey et al. 1988b, 1990; Mayhew et al. 1991). Only few studies reported the delivery of asymptomatic pups in the first litter, while pups in the second litter suffered from clinical neosporosis (Jacobson and Jardine 1993; Wouda et al. 1993). In the case described in this study, the Doberman bitch and all of its offspring from litters A and B were asymptomatic. It was not until 3 years after litter A was born that one infected pup of litter C developed clinical neosporosis, whereas a seropositive sibling of this pup was asymptomatic and the five other available siblings were seronegative for N. caninum. According to reports from the owners, all seven siblings of pup C15 did not show any neuromuscular symptoms at least up to an age of 11 months.
Postmortem examination including histology, immunohistochemistry, polymerase chain reaction of brain material, and parasite isolation in cell culture (Heckeroth et al. 2000) confirmed an active infection with N. caninum in pup C15. It is unknown why some pups develop clinical neosporosis, whereas others are infected but remain asymptomatic. In the Doberman family described in this study, 6 of 12 pups examined from a total of 22 pups delivered by the bitch were positive for N. caninum antibodies in the IFAT. The bitch had an antibody titer of 1:640, while antibody titers of its seropositive offspring varied between 1:40 and 1:640. By contrast, the antibody titer of the pup with clinical neosporosis was much higher (1:5,120).
Barber and Trees (1998) showed that bitches with latent N. caninum infection may have IFAT titers ranging from 1:50 to 1:12,800. In their study, bitches with high antibody titers (1:12,800) were estimated to transmit N. caninum to 35–96% of their offspring, with high incidence of disease in pups. Bitches with antibody titers between 1:200 and 1:800 had a placental transmission rate of 20–44%, whereas bitches with lower antibody titers transmitted the parasite to only 4–14% of their offspring, which in most cases were asymptomatic. Therefore, the authors recommended that seropositive bitches should be excluded from breeding because of a high risk of vertical transmission of N. caninum and development of congenital neosporosis in their offspring. Assuming a vertical transmission of N. caninum to all IFAT-positive pups described in this study, the placental transmission rate of the Doberman bitch (IFAT titer 1:640) was at least 27% (6 of 22). Although the true transmission rate may have even been higher, because serum samples were available for only 12 of the 22 pups delivered by the bitch, the results obtained in this study support the estimates of Barber and Trees (1998). Because it was likely for the Doberman bitch to transmit the parasite to subsequent litters, it was no longer used for breeding.
To investigate and compare the antibody responses of the IFAT-positive asymptomatic dogs with those of the pup with clinical neosporosis, we used Western blots of N. caninum Nc-1 strain tachyzoite extracts resolved by 1D or 2D PAGE under denaturing conditions. On Western blots derived from 1D PAG, five major antigenic polypeptides (mol. wt. 17,000, 30,000, 33,000, 42,000, and 48,000) were recognized by sera from IFAT-positive dogs. Western blot reactions of sera derived from IFAT-positive asymptomatic dogs were observed for all of those antigens albeit with different intensities. By contrast, the serum obtained from the pup with clinical neosporosis did not react with the 17,000, 30,000, and 33,000 mol. wt. antigenic polypeptides. This finding is interesting because this pup had the highest IFAT titer of all dogs of the Doberman family. However, it needs to be considered that while the IFAT detects antibodies directed against predominantly tachyzoite surface antigens, the extracts used in this study for preparation of Western blots also included soluble internal antigens of tachyzoites. Hence, these results show that the level of antibodies to N. caninum in dogs varies with the antigen preparation and method used for antibody detection and is not necessarily associated with disease.
Some antigens of similar size to the antigenic polypeptides detected in this study were also recognized by canine sera in other studies (Bjerkås et al. 1994; Schares et al. 2001b; Basso et al. 2005; Pinheiro et al. 2005). Basso et al. (2005) reported a case of clinical neosporosis in a 2-month-old Boxer pup that also had a high IFAT antibody titer (1:12,800). Serum obtained from this Boxer pup recognized three major antigens at 30, 37, and 45 kDa and five minor antigens at 28, 29, 43, 47, and 67 kDa. Schares et al. (2001b) developed a Western blot test to diagnose infections with N. caninum in dogs. They regard their test positive when at least two of five major antigens (i.e., 17, 29, 30, 33, and 37 kDa) are recognized by the serum. However, from the results presented in this study, where a dog suffering from clinical neosporosis developed antibodies different to those developed by asymptomatic dogs resulting in different patterns of antigenic polypeptides and fewer Western blot bands, it appears that the accuracy of Western blots for diagnosis of neosporosis must be more rigorously assessed. In addition, there is a need for standardization of antigen preparations as well as PAGE and Western blot procedures to enable the comparison of antigenic polypeptides described in different studies.
For the first time, we used Western blots derived from 2D PAG to obtain more information about antigens that are recognized by sera from IFAT-positive asymptomatic dogs and sera from dogs with clinical neosporosis. Even over a longer period of time, recognition of antigenic spots was highly reproducible when sera from asymptomatic dogs were used. For example, the pattern of antigenic spots that was recognized by the sera of the Doberman bitch did not change over a period of 2 1/4 years. Therefore, this method can be regarded as very reliable and reproducible and is thus accurate for comparison of immunoanalysis of dogs infected with N. caninum. In addition, these results suggest that the composition of antibodies in dogs with latent N. caninum infection is stable and is unlikely to change over a period of several years.
Despite its high IFAT titer, the serum of the pup with clinical neosporosis recognized only a few antigens on the Western blots derived from 2D PAG. It is interesting to note that a single antigenic spot of a mol. wt. of about 17,000 and a pI of about 4.5 was detected by this serum but was not detected by any serum obtained from IFAT-positive asymptomatic dogs. Conversely, most of the antigenic spots that were detected by sera derived from IFAT-positive asymptomatic dogs were not detected by the serum of the pup with clinical neosporosis.
Thus far, most serological reports on canine neosporosis have focused on asymptomatic dogs. Cases of congenital neosporosis are mostly diagnosed upon postmortem examination, and hence only very few sera have been collected from dogs with confirmed congenital neosporosis. However, a higher number of sera derived from dogs suffering from acute neosporosis is needed to verify the existence of antibodies to the dominant antigen detected by the Doberman pup in other pups with clinical neosporosis. To investigate this antigen, further studies shall focus on its identification, characterization, and function.
References
Almería S, Ferrer D, Pabón M, Castellà J, Mañas S (2002) Red foxes (Vulpes vulpes) are a natural intermediate host of Neospora caninum. Vet Parasitol 107:287–294
Barber JS, Trees AJ (1996) Clinical aspects of 27 cases of neosporosis in dogs. Vet Rec 139:439–443
Barber JS, Trees AJ (1998) Naturally occurring vertical transmission of Neospora caninum in dogs. Int J Parasitol 28:57–64
Barber JS, Gasser RB, Ellis J, Reichel MP, McMillan D, Trees AJ (1997) Prevalence of antibodies to Neospora caninum in different canid populations. J Parasitol 83:1056–1058
Basso W, Venturini L, Venturini MC, Hill DE, Kwok OCH, Shen SK, Dubey JP (2001) First isolation of Neospora caninum from the feces of a naturally infected dog. J Parasitol 87:612–618
Basso W, Venturini MC, Bacigalupe D, Kienast M, Unzaga JM, Larsen A, Machuca M, Venturini L (2005) Confirmed clinical Neospora caninum infection in a boxer puppy from Argentina. Vet Parasitol 131:299–303
Bjerkås I, Dubey JP (1991) Evidence that Neospora caninum is identical to the Toxoplasma-like parasite of Norwegian dogs. Acta Vet Scand 32:407–410
Bjerkås I, Mohn SF, Presthus J (1984) Unidentified cyst-forming sporozoon causing encephalomyelitis and myositis in dogs. Z Parasitenkd 70:271–274
Bjerkås I, Jenkins M, Dubey JP (1994) Identification and characterization of Neospora caninum tachyzoites antigens useful for diagnosis of neosporosis. Clin Diag Lab Immunol 1:214–221
Burkhardt E, Dubey JP, Korte G, Bauer C (1992) Zwei Erkrankungen infolge einer Infektion mit Neospora caninum bei Hundewelpen in Deutschland. Kleintierpraxis 37:701–706
Buxton D, Maley SW, Pastoret PP, Brochier B, Innes EA (1997) Examination of red foxes (Vulpes vulpes) from Belgium for antibody to Neospora caninum and Toxoplasma gondii. Vet Rec 141:308–309
Cummings JF, de Lahunta A, Suter MM, Jacobson RH (1988) Canine protozoan polyradiculoneuritis. Acta Neuropathol 76:46–54
Dubey JP (1999) Recent advances in Neospora and neosporosis. Vet Parasitol 84:349–367
Dubey JP, Lindsay DS (1996) A review of Neospora caninum and neosporosis. Vet Parasitol 67:1–59
Dubey JP, Carpenter JL, Speer CA, Topper MJ, Uggla A (1988a) Newly recognized fatal protozoan disease of dogs. J Am Vet Med Assoc 192:1269–1285
Dubey JP, Hattel AL, Lindsay DS, Topper MJ (1988b) Neonatal Neospora caninum infection in dogs: isolation of the causative agent and experimental transmission. J Am Vet Med Assoc 193:1259–1263
Dubey JP, Koestner A, Piper RC (1990) Repeated transplacental transmission of Neospora caninum in dogs. J Am Vet Med Assoc 197:857–860
Dubey JP, Dorough KR, Jenkins MC, Liddell S, Speer CA, Kwok OC, Shen SK (1998) Canine neosporosis: clinical signs, diagnosis, treatment and isolation of Neospora caninum in mice and cell culture. Int J Parasitol 28:1293–1304
Flagstad A, Jensen HE, Bjerkas I, Rasmussen K (1995) Neospora caninum infection in a litter of Labrador Retriever dogs in Denmark. Acta Vet Scand 36:387–391
Gondim LFP, McAllister MM, Mateus-Pinilla NE, Pitt WC, Mech LD, Nelson ME (2004) Transmission of Neospora caninum between wild and domestic animals. J Parasitol 90:1361–1365
Heckeroth AR, Leschke-Ramcke B, Peters M, Wohlsein P, Tenter AM (2000) A case of neosporosis in Germany. 19th conference of the German Society of Parasitology, Stuttgart, p 98
Jacobson LS, Jardine JE (1993) Neospora caninum infection in three Labrador littermates. J South Afr Vet Assoc 64:47–51
Jardine JE, Dubey JP (1992) Canine neosporosis in South Africa. Vet Parasitol 44:291–294
Lindsay DS, Dubey JP (2000) Canine neosporosis. J Vet Parasitol 14:1–11
Lindsay DS, Kelly EJ, McKown RD, Stein FJ, Plozer J, Herman J, Blagburn BL, Dubey JP (1996) Prevalence of Neospora caninum and Toxoplasma gondii antibodies in coyotes (Canis latrans) and experimental infections of coyotes with Neospora caninum. J Parasitol 82:657–659
Mayhew IG, Smith KC, Dubey JP, Gatward LK, McGlennon NJ (1991) Treatment of encephalomyelitis due to Neospora caninum in a litter of puppies. J Small Anim Pract 32:609–612
McAllister MM, Dubey JP, Lindsay DS, Jolley WR, Wills RA, McGuire AM (1998) Dogs are definitive hosts of Neospora caninum. Int J Parasitol 28:1473–1478
Munday BL, Dubey JP, Mason RW (1990) Neospora caninum infection in dogs. Aust Vet J 67:76–77
Patitucci AN, Alley MR, Jones BR, Charleston WAG (1997) Protozoal encephalomyelitis of dogs involving Neospora caninum and Toxoplasma gondii in New Zealand. N Z Vet J 45:231–235
Pinheiro AM, Costa MF, Paule B, Vale V, Ribeiro M, Nascimento I, Schaer RE, Almeida MAO, Meyer R, Freire SM (2005) Serologic immunoreactivity to Neospora caninum antigens in dogs determined by indirect immunofluorescence, western blotting and dot-ELISA. Vet Parasitol 130:73–79
Reichel MP, Thornton RN, Morgan PL, Mills RJM, Schares G (1998) Neosporosis in a pup. N Z Vet J 46:106–110
Rühlmann D, Podell M, Oglesbee M, Dubey JP (1995) Canine neosporosis: a case report and literature review. J Am Anim Hosp Assoc 31:174–183
Schares G, Wenzel U, Müller T, Conraths FJ (2001a) Serological evidence for naturally occurring transmission of Neospora caninum among foxes (Vulpes vulpes). Int J Parasitol 31:418–423
Schares G, Heydorn AO, Cüppers A, Conraths FJ, Mehlhorn H (2001b) Cyclic transmission of Neospora caninum: serological findings in dogs shedding oocysts. Parasitol Res 87:873–877
Van Ham LML, Thoonen H, Barber JS, Trees AJ, Polis I, De Cock H, Hoorens JK (1996) Neospora caninum infection in the dog: typical and atypical cases. Vlaams Diergeneeskd Tijdschr 65:326–335
Wouda W, de Jong JK, van Knapen F, Walvoort HC (1993) Neospora caninum as a cause of paralysis in littermate pups. Tijdschr Diergeneeskd 118:397–401
Acknowledgments
We gratefully acknowledge the excellent technical assistance of Angelika Duttmann, Henning Metfies, and Margret Werr. The study was supported by a scholarship of the Karl-Enigk-Stiftung to ARH. We declare that the experiments described in this study comply with current German laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Heckeroth, A.R., Tenter, A.M. Immunoanalysis of three litters born to a Doberman bitch infected with Neospora caninum . Parasitol Res 100, 837–846 (2007). https://doi.org/10.1007/s00436-006-0328-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0328-3