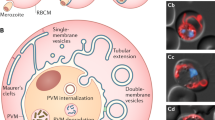
Abstract
Blood stages of Plasmodium vivax induce the development of caveolae and caveola–vesicle complexes (CVC) in the membrane of their host erythrocyte. Caveolae are found in almost all types of cells and are involved in endogenous processes as calcium and cholesterol homeostasis, cell signalling, transporting, ligand internalization and transcytosis of serum components. Major structural components of caveolae are the proteins caveolins and flotillins. The functional role of caveolae in the P. vivax-infected erythrocyte is not properly understood. As these organelles have been shown to contain malaria antigens, it has been suggested that they are involved in the transport and release of specific parasite antigens from the infected erythrocyte and in the uptake of plasma proteins. Using specific antibodies to classical caveolae proteins and an immunolocalization approach, we found caveolin-2, caveolin-3, and flotillin-2 in the vesicle profiles and some CVC of P. vivax-infected erythrocytes. Caveolin-1–3 were not found in uninfected erythrocytes. This is the first report of identification and localization of caveolins in the CVC present in erythrocytes infected with P. vivax, thereby providing evidence of the role of this particular organelle in the protein-trafficking pathway that connect parasite-encoded proteins with the erythrocyte cytoplasm and the cell surface throughout the asexual blood cycle of vivax malaria parasite.




Similar content being viewed by others
References
Adisa A, Rug M, Klonis N, Foley M, Cowman AF, Tilley L (2002) The signal sequence of exported protein-1 directs the green fluorescent protein to the parasitophorous vacuole of transfected malaria parasites. J Biol Chem 278:6532–6542
Aikawa M, Atkinson CT (1990) Immunoelectron microscopy of parasites. Adv Parasitol 29:151–214
Aikawa M, Miller LH, Rabbege J (1975) Caveola-vesicle complexes in the plasmalemma of erythrocytes infected by Plasmodium vivax and P. cynomolgi. Am J Pathol 79:285–294
Andrysiak PM, Collins WE, Campbell GH (1986) Concentration of Plasmodium ovale and Plasmodium vivax-infected erythrocytes from non-human primate blood using Percoll gradients. Am J Trop Med Hyg 35:251–254
Atkinson CT, Aikawa M (1990) Ultrastructure of malaria-infected erythrocytes. Blood Cells 16:351–368
Barnwell JW (1990) Vesicle-mediated transport of membrane and proteins in malaria-infected erythrocytes. Blood Cells 16:379–395
Barnwell JW, Ingravallo P, Galinski MR, Matsumoto Y, Aikawa M (1990) Plasmodium vivax: malarial proteins associated with the membrane-bound caveola–vesicle complexes and cytoplasmic cleft structures of infected erythrocytes. Exp Parasitol 70:85–99
Behari R, Haldar K (1994) Plasmodium falciparum: protein localization along a novel, lipid-rich tubovesicular membrane network in infected erythrocytes. Exp Parasitol 79:250–259
Bender F, Montoya M, Monardes V, Leyton L, Quest AF (2002) Caveolae and caveolae-like membrane domains in cellular signaling and disease: identification of downstream targets for the tumor suppressor protein caveolin-1. Biol Res 35:151–167
Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF (1997) Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem 272:13793–13802
Bracho C, Dunia I, Romano M, Benedetti EL, Perez HA (2001) Plasmodium vivax and Plasmodium chabaudi: intraerythrocytic traffic of homologous proteins involves a Brefeldin A-sensitive secretory pathway. Eur J Cell Biol 80:164–170
Bracho C, Dunia I, De La Rosa M, Benedetti EL, Perez HA (2002) Traffic pathways of Plasmodium vivax antigens during intraerythrocytic development. Parasitol Res 88:253–258
Brazer SW, Singh BB, Liu X, Swaim W, Ambudkar IS (2003) Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem 278:27208–27215
Edgar AJ, Polak JM (2001) Flotillin-1: gene structure: cDNA cloning from human lung and the identification of alternative polyadenylation signals. Int J Biochem Cell Biol 33:53–64
Elmendorf HG, Haldar K (1993) Secretory transport in Plasmodium. Parasitol Today 9:98–102
Field MC, Gabernet-Castello C, Dacks JB (2006) Reconstructing the evolution of the endocytic system: insights from genomics and molecular cell biology. In: Jékely G (ed) Origin and evolution of eukaryotic endomembranes and cytoskeleton. Taylor and Francis Group, Oxford, pp 1–13
Fujioka H, Torii M, Barnwell JW, Aikawa M (1992) Localization of F-actin on the caveola-vesicle complex of the erythrocytes infected with Plasmodium vivax and Plasmodium cynomolgi. Jpn J Parasitol 41:361–364
Hiller NL, Akompong T, Morrow JS, Holder AA, Haldar K (2003) Identification of a stomatin orthologue in vacuoles induced in human erythrocytes by malaria parasites. A role for microbial raft proteins in apicomplexan vacuole biogenesis. J Biol Chem 278:48413–48421
Ihalamulla RL, Mendis KN (1987) Plasmodium vivax: isolation of mature asexual stages and gametocytes from infected human blood by colloidal silica (Percoll) gradient centrifugation. Trans R Soc Trop Med Hyg 81:25–28
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Lauer S, VanWye J, Harrison T, McManus H, Samuel BU, Hiller NL, Mohandas N, Haldar K (2000) Vacuolar uptake of host components, and a role for cholesterol and sphingomyelin in malarial infection. EMBO J 19:3556–3564
Lee H, Park DS, Wang XB, Scherer PE, Schwartz PE, Lisanti MP (2002) Src-induced phosphorylation of caveolin-2 on tyrosine 19. J Biol Chem 277:34556–34567
Monier S, Parton RG, Vogel F, Behlke J, Henske A, Kurzchalia TV (1995) VIP21-caveolin, a membrane protein constituent of the caveolar coat, oligomerizes in vivo and in vitro. Mol Biol Cell 6:911–927
Monier S, Dietzen DJ, Hastings WR, Lublin DM, Kurzchalia TV (1996) Oligomerization of VIP21-caveolin in vitro is stabilized by long chain fatty acylation or cholesterol. FEBS Lett 388:143–149
Murata M, Peranen J, Schreiner R, Wieland F, Kurzchalia TV, Simons K (1995) VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci U S A 92:10339–10343
Murphy SC, Samuel BU, Harrison T, Speicher KD, Speicher DW, Reid ME, Prohaska R, Low PS, Tanner MJ, Mohandas N, Haldar K (2003) Erythrocyte detergent-resistant membrane proteins: their characterization and selective uptake during malarial infection. Blood 103:1920–1928
Nagao E, Seydel KB, Dvorak JA (2002) Detergent-resistant erythrocyte membrane rafts are modified by a Plasmodium falcuparum infection. Exp Parasitol 102:57–59
Nomura R, Fujimoto T (1999) Tyrosine-phosphorylated caveolin-1: immunolocalization and molecular characterization. Mol Biol Cell 10:975–986
Parton RG (1996) Caveolae and caveolins. Curr Opin Cell Biol 8:542–548
Pelkmans L, Helenius A (2002) Endocytosis via caveolae. Traffic 3:311–320
Perez HA, Wide A, Bracho C, De La Rosa M (1995) Plasmodium vivax: detection of blood parasites using fluorochrome labelled monoclonal antibodies. Parasite Immunol 17:305–312
Perez HA, Bracho C, Romano M, De La Rosa M (1997) Plasmodium vivax and Plasmodium chabaudi: interspecies cross-reacting antigens. Parasitol Res 83:246–251
Raposo G, Kleijmeer MJ, Posthuma G, Slot JW, Geuze HJ (1997) Immunogold labeling of ultrathin cryosections: application in immunology. Handbook of Exp Immunol 5th edn., vol. 4. Blackwell Science, Cambridge, Massachusetts, pp 1–11
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Richards W, Williams G (1973) The removal of leukocytes from malaria infected blood. Ann Trop Med Parasitol 67:249–250
Salzer U, Prohaska R (2001) Stomatin, flotillin-1, and flotillin-2 are major integral proteins of erythrocyte lipid rafts. Blood 97:1141–1143
Salzer U, Hinterdorfer P, Hunger U, Borken C, Prohaska R (2002) Ca++-dependent vesicle release from erythrocytes involves stomatin-specific lipid rafts, synexin (annexin VII), and sorcin. Blood 99:2569–2577
Sando GN, Zhu H, Weis JM, Richman JT, Wertz PW, Madison KC (2003) Caveolin expression and localization in human keratinocytes suggest a role in lamellar granule biogenesis. J Invest Dermatol 120:531–541
Sargiacomo M, Scherer PE, Tang Z, Kubler E, Song KS, Sanders MC, Lisanti MP (1995) Oligomeric structure of caveolin: implications for caveolae membrane organization. Proc Natl Acad Sci U S A 92:9407–9411
Sharma DK, Choudhury A, Singh RD, Wheatley CL, Marks DL, Pagano RE (2003) Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J Biol Chem 278:7564–7572
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A 74:4350–4354
Trelka DP, Schneider TG, Reeder JC, Taraschi TF (2000) Evidence for vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. Mol Biochem Parasitol 106:131–145
Udagama PV, Atkinson CT, Peiris JSM, David PH, Mendis KN, Aikawa M (1988) Immunoelectron microscopy of Schüffner’s dots in Plasmodium vivax-infected human erythrocytes. Am J Pathol 131:48–52
Volonte D, Galbiati F, Li S, Nishiyama K, Okamoto T, Lisanti MP (1999) Flotillins/cavatellins are differentially expressed in cells and tissues and form a hetero-oligomeric complex with caveolins in vivo. Characterization and epitope-mapping of a novel flotillin-1 monoclonal antibody probe. J Biol Chem 274:12702–12709
Acknowledgements
Thanks to the personnel of the Malaria Diagnostic Centres sponsored by the Ministry of Health and Social Development in Puerto Ayacucho, Amazonas State (Zone XX) and Caracas (Zone X) for the valuable help with the field collection of human blood samples infected with P. vivax. This research received financial support from FONACIT (grants S1-2000000633 and F-2000001531), the “Centre National de la Recherche Scientific” (CNRS, France), and ECOS-NORD-FONACIT (Grant V04S02-PI-2003000313). We assure that the experiments presented in this paper comply with the current laws of Venezuela and France.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bracho, C., Dunia, I., Romano, M. et al. Caveolins and flotillin-2 are present in the blood stages of Plasmodium vivax . Parasitol Res 99, 153–159 (2006). https://doi.org/10.1007/s00436-006-0139-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-006-0139-6