Abstract
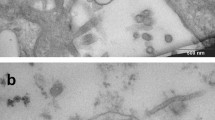
Penetration of the heteroxenous protozoan Trypanosoma rangeli into the salivary glands of its invertebrate host Rhodnius domesticus has been investigated here using different approaches. Electron microscopy showed that epimastigotes coming from the insect hemocoel cross the basal lamina that surrounds the salivary glands and penetrate through the gland cells cytoplasm. After reaching the gland lumen, epimastigote forms remain adhered to the gland cell microvilli by their flagella, while metacyclic trypomastigotes are found swimming free in the saliva. Analysis by flow cytometry, western blotting and hemolytic activity allowed to demonstrate the presence in T. rangeli of a hemolytic molecule with antigenic cross-reactivity with murine perforin, which could be used by the parasites to reach the salivary gland lumen. This molecule, which we named as rangelysin, has 120 kDa molecular weight, is able to induce hemolysis only in acidic pH, and is produced by both trypomastigote and epimastigote forms.







Similar content being viewed by others
References
Añez N (1984) Studies on Trypanosoma rangeli Tejera, 1920. VII—its effect on the survival of infected triatomine bugs. Mem Inst Oswaldo Cruz 79:249–255
Acosta L, Romanha AJ, Cosenza H, Krettli AU (1991) Trypanosomatid isolates from Honduras: differentiation between Trypanosoma cruzi and Trypanosoma rangeli. Am J Trop Med Hyg 44:676–683
Andrews NW (1990) The acid-active hemolysin of Trypanosoma cruzi. Exp Parasitol 71:241–244
Andrews NW, Portnoy DA (1994) Cytolysins from intracellular pathogens. Trends Microbiol 2:261–263
Andrews NW, Abrams CK, Slatin SL, Grifiths G (1990) A T. cruzi-secreted protein immunologically related to the complement component C9: evidence for membrane pore-forming activity at low pH. Cell 61:1277–1287
Basseri HR, Tew IF, Ratcliffe NA (2002) Identification and distribution of carbohydrate moieties on the salivary glands of Rhodnius prolixus and their possible involvement in attachment/invasion by Trypanosoma rangeli. Exp Parasitol 100:226–234
Bhakdi S, Tranun-Jensen J (1988) Damage to cell membranes by pore-forming bacterial cytolysins. Prog Allergy 40:1–43
Bielecki J, Youngman PC, Portnoy DA (1990) Bacillus subtilis expressing a haemolysin gene from Listeria monocytogenes can grow in mammalian cells. Nature 345:175–176
Carruthers VB (1999) Armed and dangerous: Toxoplasma gondii uses an arsenal of secretory proteins to infect host cells. Parasitol Internat 48:1–10
Cuba-Cuba CA (1998) Review of biological and diagnostic aspects of Trypanosoma (Herpetosoma) rangeli. Rev Soc Brasil Med Trop 31:207–220
D’Alessandro A (1976) The biology of Trypanosoma (Herpetosoma) rangeli. In: Lumsden WHR, Evans DA (eds) Biology of kinetoplastida, vol 1. Academic Press, London, pp 328–403
D’Alessandro A, Saravia NG (1992) Trypanosoma rangeli. In: Kreier J, Baker JR (eds) Parasitic protozoa, vol. 2. Academic Press, New York, pp 1–45
D’Alessandro A, Saravia NG (1999) Trypanosoma rangeli. In: Gilles HM (ed) Protozoal diseases. Arnold, London
Desai SA, Rosenberg RL (1997) Pore size of the malaria parasite’s nutrient channel. Proc Natl Acad Aci USA 94:2045–2049
Ellis DS, Evans DA, Stanford S (1980) The penetration of the salivary glands of Rhodnius prolixus by Trypanosoma rangeli. Z Parasitenk 62:63–73
Fiori PL, Rappelli P, Addis MF, Sechi A, Cappuccinelli P (1996) Trichomonas vaginalis haemolysis: pH regulates a contact-independent mechanism based on pore-forming proteins. Microb Pathog 20:109–118
Forbes MS, Plantholt BA, Sperelakis N (1977) Cytochemical staining procedures selective for sarcotubular systems of muscle: modifications and applications. J Ultrastruct Res 60:306–327
Garnham PCC (1980) The significance of inapparent infections in Chagas’ disease and other forms of trypanosomiasis. Mem Inst Oswaldo Cruz 75:181–188
Grewal MS (1957) Pathogenicity of Trypanosoma rangeli Tejera, 1920 in the invertebrate host. Exp Parasitol 6:123–130
Grisard EC (2002) Salivaria or stercoraria? The Trypanosoma rangeli dilemma. Kinetoplastid Biol Dis 1:5
Grisard EC, Steindel M, Guarneri AA, Eger-Mangrich L, Campbell DA, Romanha AJ (1999) Characterization of Trypanosoma rangeli strains isolated in Central and South America: an overview. Mem Inst Oswaldo Cruz 94:203–209
Guhl F, Vallejo GA (2003) Trypanosoma (Herpetosoma) rangeli Tejera, 1920—an updated review. Mem Inst Oswaldo Cruz 98:435–442
Guhl F, Jaramillo C, Carranza JC, Vallejo GA (2002) Molecular characterization and diagnosis of Trypanosoma cruzi and T. rangeli. Arch Med Res 33:362–370
Hecker H, Schwarzenbach M, Rudin W (1990) Development and interaction of Trypanosoma rangeli in and with reduviid bug Rhodnius prolixus. Parasitol Res 76:311–318
Hoare CA (1972) The trypanosomes of mammals. Blackwell Scientific, Oxford-Edinburgh, pp 288–314
Horstmann RD, Leippe M, Tannich E (1992) Host tissue destruction by Entamoeba histolytica: molecules mediating adhesion, cytolysis and proteolysis. Mem Inst Oswaldo Cruz 87:57–60
Horta MF (1997) Pore forming proteins in pathogenic protozoan parasites. Trends Microbiol 5:363–366
Jiang SB, Ojcius DM, Young JD (1990) Perforin binding to cells and lipid membranes determined by a simple competition assay. J Immunol Meth 126:29–37
Koerich LB, Emmanuelle-Machado P, Santos K, Grisard EC, Steindel M (2002) Differentiation of Trypanosoma rangeli: high production of infective trypomastigote forms in vitro. Parasitol Res 88:21–25
Laemmli VK (1970) Most commonly used discontinuous buffer system for SDS electrophoresis. Nature 227:680
Ley V, Robbins ES, Nussenzweig V, Andrews NW (1990) The exit of Trypanosoma cruzi from the phagosome is inhibited by raising the pH of acidic compartments. J Exp Med 171:401–413
Liu C-C, Walsh CM, Young JD-E (1995) Perforin: structure and function. Immunol Today 16:194–201
Ludwig A (1996) Cytolytic toxins from gram-negative bacteria. Microbiologia 12:281–296
Lynch EC, Rosenberg IM, Gitler C (1982) An ion-channel forming protein produced by Entamoeba histolytica. EMBO J 1:801–804
Molyneux DH, Wallbanks KR, Ingram GA (1987) Trypanosomatids-vector interfaces—in vitro studies on parasite substrate interactions. In: Chang KP, Snary D (eds) Host-parasite cellular and molecular interactions in protozoal infections, vol H11, Springer, Berlin Heidelberg New York
Nickel R, Ott C, Dandeka T, Leippe M (1999) Pore-forming peptides of Entamoeba dispar—similarity and divergence to amoebapores in structure, expression and activity. Eur J Biochem 265:1002–1007
Noronha FSM, Ramalho-Pinto FJ, Horta MF (1994) Identification of a putative pore-forming hemolysin active at acid pH in Leishmania amazonensis. Braz J Med Biol Res 27:477–482
Noronha FSM, Ramalho-Pinto FJ, Horta MF (1996) Cytolitic activity in the genus Leishmania: involvement of a putative pore-forming protein. Infect Immun 64:3975–3982
Oliveira MA, De Souza W (2001) An electron microscopic study of penetration by Trypansoma rangeli into midgut cells of Rhodnius prolixus. J Invert Pathol 77:22–26
Osorio Y, Travi BL, Palma GI, Saravia NG (1995) Infectivity of Trypanosoma rangeli in a promonocytic mammalian cell line. J Parasitol 96:449–460
Schaub GA, Wunderlich F (1985) Die chagas krankheit. Biol Med Bull 41:187–194
Shahabuddin M, Pimenta PFP (1998) Plasmodium parasites selectively invade vesicular ATPase-expressing cells in mosquito midgut. Proc Natl Acad Sci USA 95:3385–3389
Steindel M, Carvalho-Pinto C, Toma HK, Mangia RH, Ribeiro-Rodrigues R, Romanha A (1991) Trypanosoma rangeli (Tejera 1920) isolated from a sylvatic rodent (Echmys dasythrix) in Santa Catarina Island, Santa Catarina State: first report of this trypanosome in Southern Brazil. Mem Inst Oswaldo Cruz 86:73–79
Suss-Toby E, Zimmerberg J, Ward GE (1996) Toxoplasma invasion: the parasithophorous vacuole is formed from host cell plasma membrane and pinches off via a fission pore. Proc Natl Acad Sci USA 93:8413–8418
Tejera E (1920) Un noveau flagellé de Rhodnius prolixus, Trypanosoma (ou Crihtidia) rangeli n. sp. Bull Soc Pathol Exot 13:527–530
Tieszen KL, Molyneux DH, Abdel-Hafez K (1986) Host–parasite relationships of Blastocrithidia familiaris in Ligaeus pandurus Scop. (Hemiptera-Lygaeidae). Parasitology 92:1–12
Tieszen KL, Molyneux DH, Abdel-Hafez K (1989) Host-parasite relationships and cysts of Leptomonas lygaei (Trypanosomatidae) in Lygaeus pandurus (Hemiptera: Lygaeidae). Parasitology 98:395–400
Tobie EJ (1965) Biological factors influencing transmission of Trypanosoma rangeli by Rhodnius prolixus. J Parasitol 51:837–841
Tweten RK (1995) In: Roth JA et al. (eds) Virulence mechanisms of bacterial pathogens. ASM Press, pp 207–229
Watkins R (1971) Histology of Rhodnius prolixus infected with Trypanosoma rangeli. J Invert Pathol 117:59–66
Young JDE, Young TM, LU LP, Unkelles JC, Cohn ZA (1982) Characterization of a membrane pore-forming protein from Entamoeba histolytica. J Exp Med 156:1677–1690
Acknowledgements
The authors thank Dr. Pedro M. Persechini (Institute of Biophysics, UFRJ, Rio de Janeiro, Brazil) for kindly supplying the anti-perforin antibody and Mr. José Lopes de Faria for the photographic work. This work was supported by CNPq, FAPERJ, PAPES-III/FIOCRUZ and FIOCRUZ. All experiments were performed according to the Brazilian laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meirelles, R.M.S., Henriques-Pons, A., Soares, M.J. et al. Penetration of the salivary glands of Rhodnius domesticus Neiva & Pinto, 1923 (Hemiptera: Reduviidae) by Trypanosoma rangeli Tejera, 1920 (Protozoa: Kinetoplastida). Parasitol Res 97, 259–269 (2005). https://doi.org/10.1007/s00436-005-1433-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-1433-4