Abstract
A total of 113 mentally retarded patients residing in a mental institution in Metropolitan Manila, Philippines, were screened for the presence of Entamoeba histolytica based on microscopy and polymerase chain reaction (PCR). Anti-E. histolytica antibodies were also screened in 97 serum samples collected using immunofluorescence antibody (IFA) test. Parasitological examination showed E. histolytica/Entamoeba dispar in 43 cases (38.05%), while PCR detected 74 cases (65.48%) positive for E. histolytica and 6 cases (5.30%) positive for E. dispar. Interestingly, these 6 samples were coinfected with E. histolytica. IFA test revealed that 80.41% (78/97) of the respondents possessed significant antibody titers for intestinal infection of E. histolytica. Of this number, there were 5 patients negative in IFA test but positive in PCR. The genetic diversity of E. histolytica isolates was also investigated by analyzing polymorphism in the serine-rich gene by nested PCR on DNA directly extracted from stool specimens. A combination of the nested PCR results and the AluI digestion of the PCR products examined yielded six distinct DNA banding patterns among the 74 stool isolates. An apparent clustering of E. histolytica strains was observed in patients living in different residential cottages of the institution. These results indicate the high prevalence of E. histolytica in an institution for the mentally retarded in the Philippines.
Similar content being viewed by others
Introduction
Amebiasis is a widespread parasitic disease caused by the protozoan Entamoeba histolytica. It has been estimated that this organism causes up to 100,000 deaths per year, placing it second only to malaria due to protozoan parasites (World Health Organization 1997). The spread of disease is by fecal–oral route and is common among individuals in lower socioeconomic status because of lack of adequate sanitation. In addition, oral–anal sexual practices and long-term institutionalization with psychiatric illness or mental retardation are also established risk factors (Ravdin 1994). Recently, delineation of E. histolytica into two genetically distinct species—the pathogenic E. histolytica Schaudinn, 1903, and the nonpathogenic Entamoeba dispar Brumpt, 1925—has been established (Diamond and Clark 1993). Only E. histolytica (in the strict sense) can cause intestinal and extraintestinal diseases. In view of this development, there is a need to reassess previously reported prevalence data of E. histolytica infections in many parts of the world. Moreover, information on intraspecies variation within E. histolytica is also needed to accurately investigate the epidemiology of this organism in endemic areas.
The prevalence of E. histolytica in mental institutions has been documented in the United States (Sexton et al. 1974; Thacker et al. 1979), United Kingdom (Sargeaunt and Williams 1982), Italy (Gatti et al. 1995), Taiwan (Cheng and Wang 1999), and Japan (Nagakura et al. 1989, 1990; Tachibana et al. 2000). With the exception of three reports (Sexton et al. 1974; Thacker et al. 1979; Nagakura et al. 1989), studies done in these countries accurately distinguished E. histolytica from E. dispar using zymodeme analysis, reactivity to monoclonal antibodies, and polymerase chain reaction (PCR). However, to date, no study has been reported on the prevalence of E. histolytica in a mental institution in the Philippines. Also, there has been no report on the subtyping of E. histolytica strains using polymorphic DNA markers in mental institutions. This study was therefore undertaken to identify the species of amebae and strains of E. histolytica which are prevalent in a mental institution in Metropolitan Manila, Philippines.
Materials and methods
Study area
The mental institution we studied is located in the southern part of Metropolitan Manila. At the time of the study, 526 patients with variable degrees of mental retardation are housed in 14 separate residential cottages. Each cottage accommodates 30–40 individuals of similar gender, mental age, and estimated biological age. The first level of mental age is termed as the profound level (0–15 intelligence quotient, IQ), followed by lower prospective (16–30 IQ), lower trainable (31–45 IQ), trainable (46–60 IQ), and upper trainable (61–75 IQ). The exact age of the majority of the patients is not known since most of them are taken from the streets by various government agencies and are turned over to the institution for proper care and training. Within the premise of each housing facility, a separate dining hall with communal tables is present, along with barrack-like bedroom hall and communal toilet and bath facility. The institution is surrounded by a lawn to which the less severely affected patients have free access.
Collection of samples
One hundred thirteen stool samples (21.48%) and 97 blood specimens (18.44%) were collected from the 526 in-house patients of the institution. Particular care was taken in explaining to the employees responsible for the patients the need to label the plastic containers with name and gender and to avoid sample contact with soil and with samples from other individuals. After the collection, stool specimens were transported to the laboratory within 6 h. Each fecal sample was subjected to formalin–ether sedimentation procedure (Beaver et al. 1984). The presence of parasites was determined by microscopic examination of fresh stools as well as formalin–ether concentrated specimens. Blood samples were collected using Microtainer Safety Flow lancet and were placed into serum separator tubes. The samples were then centrifuged for 5 min at 1,200×g, and the resulting sera were frozen at −20°C until assayed for anti-human IgG antibodies (MBL Co., Nagoya, Japan).
Extraction of DNA from stool specimens
Genomic DNA was extracted from cysts present in formalin-fixed stool specimens as previously described (Rivera et al. 1996, 1998, 1999). Briefly, the pellet resulting from the sedimentation procedure was washed four times with phosphate-buffered saline. The pellet from the last wash was resuspended in TE buffer (100 mM Tris, pH 8.0, 25 mM EDTA) and subjected to freezing (using dry ice and ethanol for 6 min) and thawing (at 37°C in a water bath for 3 min) six times. After the last treatment, the solution was mixed with 200 μl of Triton X-100 (final concentration of 1%) and then heated in 98°C water bath for 10 min. The mixture was then incubated with 500 μg/ml of proteinase K in lysis buffer (100 mM Tris, pH 8.0, 1% sodium dodecyl sulfate, 25 mM EDTA) at 60°C for 2 h, and the DNA was extracted using phenol–chloroform and precipitated using ethanol.
Polymerase chain reaction
Polymerase chain reaction was carried out using primers specific for E. histolytica and E. dispar (p11 plus p12 and p13 plus p14, respectively) as previously described (Tachibana et al. 1991). Genetic diversity among E. histolytica-positive samples was further investigated by analyzing polymorphism in the serine-rich gene by nested PCR and AluI (Takara Biomedicals) digestion of PCR products as recently reported (Ayeh-Kumi et al. 2001). The PCR and the digested products were separated electrophoretically in 2% LO3 agarose (Takara Biomedicals) gels stained with ethidium bromide. The gels were visualized by UV light and photographed.
Immunofluorescence antibody test
Serology was examined by the immunofluorescence antibody (IFA) test previously described, using formalin-fixed trophozoites of E. histolytica HK-9 as antigen (Nagakura et al. 1989; Tachibana et al. 1990). Titers with 1:64 or more were scored positive.
Results
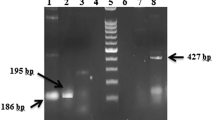
One hundred thirteen stool samples taken from the survey population were examined for the presence of common intestinal parasites by microscopy (Table 1). Of this number, 43 (38.05%) had a positive stool examination for E. histolytica/E. dispar. Stools were also assayed with PCR using specific primers, p11 plus p12 and p13 plus p14, which accurately detect E. histolytica and E. dispar, respectively (Fig. 1). Of the 113 samples analyzed, 74 (65.48%) samples were positive for E. histolytica, while only 6 (5.30%) samples produced amplification product for E. dispar primers. Interestingly, we found that these 6 samples were coinfected with E. histolytica. Moreover, of the 74 samples positive for E. histolytica, 23 (31.08%) were males and 51 (68.92%) were females. With the nested serine-rich Entamoeba histolytica protein (SREHP) PCR protocol recently described (Ayeh-Kumi et al. 2001), amplification was achieved in all E. histolytica-positive samples detected by primers p11 plus p12. This confirms the specificity of both the p11 plus p12 and nested SREHP primers in detecting E. histolytica from field-collected samples. Five distinct DNA patterns were observed using the nested SREHP PCR protocol among the 74 E. histolytica isolates (Fig. 2). However, after digestion of the nested PCR products with the restriction endonuclease AluI, four distinct patterns were obtained (Fig. 3). Combined DNA patterns of nested SREHP PCR products and AluI-digested bands showed six distinct patterns (Table 2). An apparent clustering of E. histolytica strains was observed in different cottages, but noteworthy is DNA pattern no. 6, which was observed only in cottage that houses profoundly retarded patients.
We were able to collect blood samples from 97 patients. We realize the limitation of not securing blood samples from the 113 patients who participated in this study. This was done, however, in an effort to reduce the problems involved with collection of blood samples from 16 severely handicapped patients. IFA test revealed that 80.41% (78/97) of the respondents possessed significant antibody titers for intestinal infection of E. histolytica. Of this number, there were 5 patients negative in IFA test but positive in PCR (data not shown).
Discussion
E. histolytica infections are not uncommon in institutionalized psychiatric or mentally retarded patients. Epidemiologic studies in the United States (Sexton et al. 1974; Thacker et al. 1979), United Kingdom (Sargeaunt and Williams 1982), Italy (Gatti et al. 1995), Taiwan (Cheng and Wang 1999), and Japan (Nagakura et al. 1989, 1990; Tachibana et al. 2000) revealed positive rates ranging from 2% to more than 30%. The transmission of amebiasis among these patients has been attributed not only to the direct fecal–oral route but also to their abnormal behavior.
Similarly, there are several possible factors affecting the prevalence of E. histolytica and other parasites in the study site. Mental state of the patients was observed to be closely related with the hygienic condition of the residential cottages. Cottages that house profoundly retarded patients were observed to be unsanitary. Residential cottages of trainable patients, on the other hand, were more sanitary, indicating the acquired training on the proper use of toilet and personal hygiene. However, results show that E. histolytica prevalence was still high in this mental age group. The lack of high mental capacity cannot be underestimated despite of the training the patients receive in the institution. Common behaviors of the patients such as nail biting, improper food handling, and hand-to-mouth or object-to-mouth habits greatly influence the transmission of the parasite.
Microscopic examination only detected 43 samples positive for E. histolytica/E. dispar (38.05%). Interestingly, these samples were all positive in PCR, and the corresponding serum samples were positive in IFA test. This result indicates that several cyst passers were detected only by either PCR or IFA test or both assays but not by microscopy. The PCR and IFA test used in this study are sensitive and specific assays that detect amebiasis infection. The use of both assays simultaneously in our investigation provided a more conclusive data since they established the state of infection (whether previous or current infection). PCR detects amebic antigens as a result of current infection. IFA test, on the other hand, is able to detect antibodies even years after an episode of amebiasis. Our results correlated with these facts: 30 patients positive in IFA test were found negative in PCR, indicating prior exposure of these individuals to E. histolytica. However, 5 of the 97 patients (5.15%) negative in IFA test were found positive in the PCR assay. We attribute this to the possibility that these 5 respondents were newly infected with the parasite and that the antibody levels were not sufficient enough to be detected by IFA test. Generally, IgG is elicited only after an infection is fully established. This explains the possibility of not detecting an antibody response despite the presence of an antigen in the stool sample (Haque et al. 2000). Since this study is cross-sectional sampling, we could not determine whether those PCR-positive but seronegative patients developed an antibody response later.
While majority of the patients remained asymptomatic, in general, the following clinical symptoms were observed among the 113 respondents: mild diarrhea (two to three bowel movements per day), bloody stool, mucus laden feces, abdominal pain, fever, and vomiting. Seven E. histolytica-positive patients presented with bloody stool, abdominal pain, and fever during the duration of the study. Ten of those who were negative for E. histolytica, on the other hand, presented with mild diarrhea, mucus laden stool, and occasional abdominal pains. The rest of the respondents were asymptomatic. However, a comparative study between the clinical symptoms presented by E. histolytica-positive patients and those who do not harbor the organism is inconclusive since majority of the patients suffered from multiple parasitic infections and thus produced an array of clinical symptoms.
Using the polymorphic SREHP gene (Ayeh-Kumi et al. 2001; Clark and Diamond 1993), we sought to determine the strains of E. histolytica which are prevalent in the study site. It is interesting to note that isolates from the study area exhibited considerable polymorphism in the SREHP gene. The comparison of isolates from different residential cottages was of note. DNA pattern no. 6 was found only in cottage that houses profoundly retarded patients. This mental age group has been confined to its isolated cottage for a long period without interaction with patients of other mental groups. This may probably explain the existence of this particular strain of E. histolytica in this mental age group. A more prevalent strain (DNA pattern no. 3), however, was observed in all residential cottages housing different mental age groups. These observations therefore show the promise of SREHP in providing greater understanding of the epidemiology of amebic infection not only in confined institutions but also in other endemic communities as well as in clinical isolates. Analyses of additional polymorphic loci (Zaki and Clark 2001; Zaki et al. 2002) to fully characterize possible variants within isolates may also be applied to accurately define the pathogenic potential of E. histolytica strains in endemic population.
In this study, we found that the prevalence of amebiasis in a mental institution in the Philippines is high. The aforementioned factors that exist within the boundary of the mental institution along with the lack of mental capacity pose a greater risk of acquiring amebiasis. Long-term institutionalization, therefore, is a significant aspect in the epidemiology of amebiasis. Because of this alarmingly high prevalence of E. histolytica in the study site, we advise that patients be subjected to periodic health checks to detect cases of invasive amebiasis. Moreover, newly confined individuals should also be subjected to this procedure to prevent potential further spread of the parasite in the institution.
References
Ayeh-Kumi PF, Ali IM, Lockhart LA, Gilchrist CA, Petri WA Jr, Haque R (2001) Entamoeba histolytica: genetic diversity of clinical isolates from Bangladesh as demonstrated by polymorphisms in the serine-rich gene. Exp Parasitol 99:80–88
Beaver PC, Jung RC, Cupp EW (1984) Clinical parasitology, 9th edn. Lea & Febiger, Philadelphia, 741 pp
Cheng HS, Wang LC (1999) Amoebiasis among institutionalized psychiatric patients in Taiwan. Epidemiol Infect 122:317–322
Clark CG, Diamond LS (1993) Entamoeba histolytica: a method for isolate identification. Exp Parasitol 77:450–455
Diamond LS, Clark CG (1993) A redescription of Entamoeba histolytica Schaudinn, 1903 (Emended Walker, 1911) separating it from Entamoeba dispar Brumpt, 1925. J Eukaryot Microbiol 40:340–344
Gatti S, Cevini C, Marchi L, Novati S, Scaglia M (1995) Entamoeba histolytica autochthonous isolates from mentally retarded Italian patients. Parasitol Res 81:148–151
Haque R, Mollah NU, Ali IM, Alam K, Eubanks A, Lyerly D, Petri WA Jr (2000) Diagnosis of amebic liver abscess and intestinal infection with the TechLab Entamoeba histolytica II antigen detection and antibody tests. J Clin Microbiol 38:3235–3239
Nagakura K, Tachibana H, Tanaka T, Kaneda Y, Tokunaga M, Sasao M, Takeuchi T (1989) An outbreak of amebiasis in an institution for the mentally retarded in Japan. Jpn J Med Sci Biol 42:63–76
Nagakura K, Tachibana H, Kaneda Y, Suzuki H, Sasaoka K, Kobayashi S, Takeuchi T (1990) Amebiasis in institutions for the mentally retarded in Kanagawa Prefecture, Japan. Jpn J Med Sci Biol 43:123–131
Ravdin JI (1994) Diagnosis of invasive amebiasis—time to end the morphology era. Gut 35:1018–1021
Rivera WL, Tachibana H, Silva-Tahat MRA, Uemura H, Kanbara H (1996) Differentiation of Entamoeba histolytica and E. dispar DNA from cysts present in stool specimens by polymerase chain reaction: its field application in the Philippines. Parasitol Res 82:585–589
Rivera WL, Tachibana H, Kanbara H (1998) Field study on the distribution of Entamoeba histolytica and Entamoeba dispar in the Northern Philippines as detected by the polymerase chain reaction. Am J Trop Med Hyg 59:916–921
Rivera WL, Tachibana H, Kanbara H (1999) Application of polymerase chain reaction (PCR) in the epidemiology of Entamoeba histolytica and Entamoeba dispar infections. Tokai J Exp Clin Med 23:413–415
Sargeaunt PG, Williams JE (1982) A study of intestinal protozoa including non-pathogenic Entamoeba histolytica from patients in a group of mental hospitals. Am J Public Health 72:178–180
Sexton DJ, Krogstad DJ, Spencer HC Jr, Healy GR, Sinclair S, Sledge CE, Schultz MG (1974) Amebiasis in a mental institution: serologic and epidemiologic studies. Am J Epidemiol 100:414–423
Tachibana H, Kobayashi S, Kato Y, Nagakura K, Kaneda Y, Takeuchi T (1990) Identification of a pathogenic isolate-specific 30,000-M r antigen of Entamoeba histolytica by using a monoclonal antibody. Infect Immun 58:955–960
Tachibana H, Kobayashi S, Takekoshi M, Ihara S (1991) Distinguishing pathogenic isolates of Entamoeba histolytica by polymerase chain reaction. J Infect Dis 164:825–826
Tachibana H, Kobayashi S, Nagakura K, Kaneda Y, Takeuchi T (2000) Asymptomatic cyst passers of Entamoeba histolytica but not Entamoeba dispar in institutions for the mentally retarded in Japan. Parasitol Int 49:31–35
Thacker SB, Simpson S, Gordon TJ, Wolfe M, Kimball AM (1979) Parasitic disease control in a residential facility for the mentally retarded. Am J Public Health 69:1279–1281
World Health Organization (1997) Entamoeba taxonomy. Bull World Health Organ 75:291–292
Zaki M, Clark CG (2001) Isolation and characterization of polymorphic DNA from Entamoeba histolytica. J Clin Microbiol 39:897–905
Zaki M, Meelu P, Sun W, Clark CG (2002) Simultaneous differentiation and typing of Entamoeba histolytica and Entamoeba dispar. J Clin Microbiol 40:1271–1276
Acknowledgements
We thank Miki Kinoshita, Tetsuo Yanagi, Aleyla S. Escueta, and John Anthony D.L. Yason for the technical assistance. This work was supported by a Grant-in-Aid for Scientific Research (no. 12576008) from the Ministry of Education, Science, Sports, and Culture, Japan. Windell L. Rivera was a visiting research fellow of the Institute of Tropical Medicine, Nagasaki University, Japan, at the time this study was conducted. The experiments conducted in this study comply with the current laws of the Philippines.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rivera, W.L., Santos, S.R. & Kanbara, H. Prevalence and genetic diversity of Entamoeba histolytica in an institution for the mentally retarded in the Philippines. Parasitol Res 98, 106–110 (2006). https://doi.org/10.1007/s00436-005-0024-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-005-0024-8