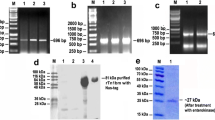
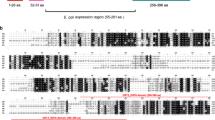
Abstract
Prior studies have demonstrated that transglutaminase (TGase) from the human filarial parasite Brugia malayi is critical for the growth and development of the larval stages. In this report, we describe the cloning and partial characterization of a cDNA encoding the B. malayi TGase (BmTGase). Using RT-PCR and RACE-PCR, the cDNA was amplified from adult worm mRNA. BmTGase is 1,881 bp long and codes for a protein with a predicted molecular mass of 54 kDa. Amino acid sequence analysis of BmTGase revealed significant homology to the protein disulfide isomerase (PDI), particularly, to the PDI-related protein ERp60, a PDI isoform found in the lumen of endoplasmic reticulum. The activity of recombinant B. malayi TGase enzyme (rBmTG) was found to be calcium-dependent and could be inhibited by EDTA. ELISA studies showed that approximately 88% of 48 sera from healthy Indian patients living in a bancroftian filariasis endemic area were reactive with rBmTG. In contrast, only 33% of sera from patients with clinical filariasis were reactive to rBmTG. Non-endemic sera were uniformly non-reactive. Additional studies are needed to elucidate the role, if any, of B. malayi TGase in protective immunity to filariasis.




Similar content being viewed by others
References
Adini A, Krugliak M, Ginsburg H, Li L, Lavie L, Warburg O (2001) Transglutaminase in Plasmodium parasites: activity and putative role in oocysts and blood stages. Mol Biochem Parasitol 117:161–168
Blasko B, Madi A, Fesus L (2003) Thioredoxin motif of Caenorhabditis elegans PDI-3 provides Cys and His catalytic residues for transglutaminase activity. Biochem Biophys Res Commun 303:1142–1147
Chandrashekar R (2001) Parasitic nematode transgluaminase, nucleic acid molecules, and uses thereof. US Patent 6,248,872
Chandrashekar R (2002). Novel parasite (nematode) transglutaminase-potential drug target. Indian J Exp Biol 40:753–754
Chandrasekar R, Mehta K (2000) Transglutaminase-catalysed reactions in the growth and development of parasitic nematodes. Parasitol Today 16:11–17
Chandrashekar R, Tsuji N, Morales T, Ozols V, Mehta K (1998) An ERp60-like protein from the filarial parasite D. immitis has both transglutaminase and protein disulfide isomerase activity. Proc Natl Acad Sci U S A 95:531–536
Chandrasekar R, Devarajan E, Mehta K (2002) D. immitis: further characterization of the transglutaminase enzyme and its role in larval molting. Parasitol Res 88:185–191
Eschenlauer SC, Page AP (2003). The Caenorhabditis elegans ERp60 homolog protein disulfide isomerase-3 has disulfide isomerase and transglutaminase-like cross-linking activity and is involved in the maintenance of body morphology. J Biol Chem 278:4227–4237
Elson LH, Guderian, RH, Araujo E, Bradley JE, Days A, Nutman TB (1994) Immunity to onchocerciasis: identification of a putatively immune population in a hyperendemic area of Ecuador. J Infect Dis 169:588
Gregory WF, Atmadja AK, Allien JE, Maizels R (2000) The abundant transcript-1 and -2 genes of B. malayi encode stage specific candidate vaccines antigens for filariasis. Infect Immun 68:4147–4179
Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, Sato Y, Saito Y (2003) A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J 373:793–803
Irvine M, Johnson EH, Lustigman S (1997) Identification of larval-stage-specific antigens of Onchocerca volvulus uniquely recognized by putative immune sera from humans and vaccination sera from animal models. Ann Trop Med Parasitol 91:67–77
Kemmink J, Darby NJ, Dijkstra K, Nilges M, Creighton TE (1997) The folding catalyst protein disulfide isomerase is constructed of active and inactive thioredoxin modules. Curr Biol 7:239–45
Keiser PB, Nutman TB (2002) Update on lymphatic filarial infections. Curr Infect Dis Rep 4:65–69
Knodler LA, Noiva R, Mehta K, McCaffery JM, Aley SB, Svard SG, Nystul TG, Reiner DS, Silberman JD, Gillin FD (1999) Novel protein-disulfide isomerases from the early-diverging protest Giardia lamblia. J Biol Chem 274:29805–29811
Lalitha P, Eswaran D, Gnansekar M, Rao KVN, Narayanan RB, Scott A, Nutman T, Kaliraj P (2002) Development of antigen detection ELISA for the diagnosis of Brugian and Bancroftian filariasis using antibodies to recombinant filarial antigens Bm-SXP-1 and Wb-SXP-1 Microbiol Immunol 46:327–332
Lizotte WM, Tawe W, Guiliano DB, Lu W, Liu J, William SA, Lustigman S (2002) Identification of potential vaccine and drug target candidates by expressed sequence tag analysis and immunoscreening of Onchocerca volvulus larval cDNA libraries. Infect Immun 68:3491–3501
Lustigman S, Brotman B, Huima T, Castelhano AL, Singh RN, Mehta K, Prince AM (1995) Transglutaminase-catalyzed reaction is important for molting of Onchocerca volvulus third-stage larvae. Antimicrob Agents Chemother 39:1913–1919
McCarthy JS, Nutman TB (1996) Perspective: prospects for development of vaccines against human helminth infections. J Infect Dis 174:1384–1390
Mehta K, Rao, UR, Vickery AC, Birckbichler PJ (1990) Significance of transglutaminase catalysed reactions in growth and development of filarial parasite, B. malayi. Biochem Biophy Res Commun 173:1051–1057
Michael E, Bundy DA, Grenfell BT (1996) Reassessing the global prevalence and distribution of lymphatic filariasis. Parasitology 112:409–428
Murray J, Gregory WF, Gomez-Escobar N, Atmadja AK, Maizels RM (2001) Expression and immune recognition of B. malayi VAL-1, a homologue of vespid venom allergens and Ancylostoma secreted proteins. Mol Biochem Parasitol 118:89–96
Natsuka S, Takubo R, Seki R, Ikura K (2001) Molecular characterization and expression of Caenorhabditis elegans ERp57-homologue with transglutaminase activity. J Biochem 130:731–735
Nutman TB, Steel C, Ward DJ, Zea-Flores G, Ottesen EA (1991) Immunity to onchocerciasis: recognition of larval antigens by human putative immune to Onchocerca volvulus infection. J Infect Dis 163:1128–1133
Rao KV, Eswaran M, Ravi V, Gnanasekhar B, Narayanan RB, Kaliraj P, Jayaraman K, Marson A, Raghavan N, Scott AL (2000) The Wuchereria bancrofti orthologue of Brugia malayi SXP1 and the diagnosis of bancroftian filariasis. Mol Biochem Parasitol 107:71–80
Rao UR, Mehta K, Subrahmanyam D, Vickery AC (1991) B. malayi and Acanthocheilonema viteae: antifilarial activity of transglutaminase inhibitors in vitro. Antimicrob Agents Chemother 35:2219–2224
Sharma S, Bhattacharyya MK (1997) Novel putative protective antigens of Plasmodium falciparum. Indian J Med Res 106:63–69
Slaughter TF, Achyuthan KE, Lai TS, Greenberg CS (1992) A microtiter plate transglutaminase assay utilizing 5-(biotinamido) pentylamine as substrate. Anal. Biochem 205:166–171
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Devarajan, E., Mishra, P.K., Thirugnanam, S. et al. Molecular characterization of a Brugia malayi transglutaminase. Parasitol Res 93, 145–150 (2004). https://doi.org/10.1007/s00436-004-1121-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-004-1121-9