Abstract
Frostius pernambucensis is a phytotelm-breeding frog with endotrophic larvae. Although the larvae were formally described, no aspect of its internal morphology is known. In this paper, we re-describe the tadpole based on a large sample, describe its internal anatomy (buccopharyngeal cavity and musculo-skeletal system), provide data on natural history, and discuss the evolution of endotrophy and phytotelma colonization. The tadpoles of F. pernambucensis are highly modified, with depressed bodies, reduced mouthparts, and long tails. Many character-states described for these tadpoles can be related to its endotrophic development. Consequence of this highly modified phenotype, we propose several novel putative synapomorphies for the genus: (1) labial tooth row formula 1/1; (2) absence of pustulation in the buccal roof and (3) floor; (4) absence of median ridge; (5) absence of lateral ridge papillae; (6) absence of secretory ridges and pores; (7) absence of filter plates; (7) m. subarcualis rectus II–IV originating on ceratobranchial III; (8) m. subarcualis rectus II–IV inserting on ceratobranchial I; (8) ventral slip of the m. subarcualis rectus I inserting on the ceratobranchial III; (9) suprarostral corpora fused to the cornua trabeculae; (10) commissura quadratoorbitalis absent; (11) cerabranchial II attached to the planum hypobranchiale; and (12) ceratobranchial III attached to the planum hypobranchiale. Finally, we also propose that the presence of a single pair of infralabial papilla could represent a synapomorphy of bufonids. The colonization of phytotelma seem to have created a selective pression on the development of F. pernambucenis, favoring the evolution of endotrophy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The species-rich family Bufonidae Gray, 1825 is a nearly cosmopolitan clade that originated in South American in the Upper Cretaceous and rapidly expanded all over the major continents except the Australo-Papuan region, Madagascar, and Antarctic (Pramuk et al. 2008; Van Bocxlaer et al. 2010; Frost 2023). Within the more than 600 known species, a relevant diversity has been reported, including a great spectrum of reproductive modes, from oviparity with lentic or lotic free-living exotrophic tadpoles, endotrophic tadpoles that develop in phytotelmata, up to viviparous species with direct development (Baldo et al. 2014; Wake 2015; Chandramouli et al. 2016; Liedtke et al. 2022).
Originally associated with the genus Atelopus Duméril & Bibron, 1841, the bufonid Frostius pernambucensis (Bokermann 1962) was described (as Atelopus pernambucensis) for a remnant of the Atlantic Forest in the municipality of Recife, state of Pernambuco, Brazil (Bokermann 1962). After two decades of the species discovery, Cruz and Peixoto (1982) described its tadpole from the type locality, as well as information on spawning and oviposition site. The species is a phytotelm-breeding frog with endotrophic larvae (Cruz and Peixoto 1982). The reproductive differences observed between F. pernambucensis and the remaining Atelopus suggested that they belong to different lineages (Cruz and Peixoto 1982). Later, Cannatella (1986), based on data on adult morphology, larvae, and spawning, proposes the genus Frostius to accommodate this species. The genus remained monotypic until the recent description of F. erythrophthalmus Pimenta & Caramaschi 2007 for the Atlantic Forest of the municipality of Uruçuca, state of Bahia, Brazil, whose tadpole remains unknown (Pimenta and Caramaschi 2007). Currently, Frostius is endemic to the Atlantic Rainforests of northeastern Brazil (Frost 2023). The genus is one of the early divergent lineages within bufonids, being the sister to all bufonids but Melanophryniscus (Jetz and Pyron 2018).
Larval morphology information proved to be very important and informative in understanding the taxonomy and the phylogenetic relationships of several anurans (e.g., Noble 1929; Orton 1953; Maglia et al. 2001; Haas 2001, 2003; Pugener et al. 2003; Dias et al. 2021, 2023), but data are lacking for several taxa. This is the case of Frostius pernambucensis; even though larval morphology was pivotal for the generic classification of the species, since the original tadpole description no further studies were performed, and aspects of its internal anatomy remain unknown. Moreover, the tadpole of F. pernambucensis was characterized based on seven individuals in early developmental stages and one egg from the same spawning raised in laboratory, and thus, several important characters were not observed or described. Recently, Dubeux et al. (2020a) provided a brief characterization for the tadpoles of F. pernambucensis for state of Alagoas, aiming a generic identification, but not addressing other morphological aspects of the tadpole. The fact that the larvae of F. pernambucensis are endotrophic (Cruz and Peixoto 1982) is contrasting with most of bufonids, making it a very intriguing piece in the puzzled evolutionary history of the family.
Given the above, a more detailed approach is needed to understand the evolution of the unique tadpoles of Frostius pernambucensis. Herein, we present a complete redescription of external morphology and the first description of internal anatomy of tadpole of this species. Additionally, we discuss the importance of larval characters in the systematics of bufonids and address the evolution of endotrophic tadpoles.
Materials and methods
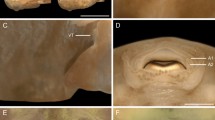
Tadpoles were collected in Atlantic Forest remnants in the state of Pernambuco and Alagoas (Fig. 1). The three first lots (MHNUFAL 14572, 16192, 16196) are from Estação Ecológica de Murici, municipality of Murici, state of Alagoas, Brazil, 167 km south-west from the type locality of species (35° 52′ 41.50" W; 9° 12′ 54.74" S, 462 m a.s.l.; Fig. 1). Tadpoles were collected in July 2015, June, and July 2021, respectively. The other two lots (MHNUFAL 17359, 17360) are from Reserva Particular do Patrimônio Natural Eco Fazenda Morim, municipality of São José da Coroa Grande, state of Pernambuco, Brazil, 99 km south from the type locality of species (8° 52′ 10.46" S, 35° 12′ 28.57" W; 92 m a.s.l.), and were collected in May 2022.
Geographic distribution of the Frostius pernambucensis highlighting the areas in which the tadpoles analyzed in this study were collected (cross and star). A Inset map: South America, highlighting the distribution of the Atlantic Forest (green) and the magnified area showed in A (gray rectangle). B Known localities of the species (diamonds) and its type locality (star). C Panoramic view of Parque Estadual Dois Irmãos, municipality of Recife, state of Pernambuco. D Panoramic view of Reserva Particular do Patrimônio Natural Eco Fazenda Morim, municipality of São José da Coroa Grande, state of Pernambuco. E Panoramic view of Estação Ecológica de Murici, municipality of Murici, state of Alagoas. List of locations and references: 1 = Paraíba: João Pessoa (Pimenta and Caramaschi 2007); 2 = Pernambuco: Paulista (Barbosa et al. 2017); 3 = Pernambuco: Recife (Bokermann 1962); 4 = Pernambuco: São José da Coroa Grande (present study); 5 = Alagoas: Ibateguara (Pimenta and Caramaschi 2007); 6 = Alagoas: Murici (Pimenta and Caramaschi 2007); 7 = Alagoas: Maceió (Dubeux et al. 2020b); 8 = Bahia: Varzedo (Juncá and Borges 2002); 9 = Bahia: São Sebastião do Passé (Freitas et al. 2019)
The analyzed specimens are deposited in the herpetological collection of the Museu de História Natural of the Universidade Federal de Alagoas (MHNUFAL). Additionally, we also analyzed the original series of larval description of the species (EI 7253; Cruz and Peixoto 1982).
External morphology
Measurements were made in 17 tadpoles in stages 37 to 41 (Gosner 1960). Five of these tadpoles (stage 38) were used for qualitative description (MHNUFAL 16192, 16196). Measurements followed Altig and McDiarmid (1999): body length (BL), maximum tail height (MTH), tail length (TaL), tail muscle height (TMH), tail muscle width (TMW), and total length (TL); Lavilla and Scrocchi (1986): body width at eye level (BWE), body width at nostril level (BWN), extranarial distance (EnD), extraorbital distance (EoD), eye diameter (ED), intranarial distance (InD), intraorbital distance (IoD), maximum body height (MBH), maximum body width (MBW), narial diameter (ND), oral disc width (ODW), snout-spiracle distance (SSD), and spiracle-posterior body distance (SPD); and Grosjean (2005): dorsal fin height (DFH) and ventral fin height (VFH).
All measurements were taken using an ocular micrometer installed on a Leica® MZ6 stereomicroscope, except for TL, which was measured with digital calipers (0.1 mm accuracy). Terminology follows Altig and McDiarmid (1999). For color descriptions, we used the terminology proposed by Köhler (2012) with their corresponding color codes.
Buccopharyngeal cavity
To study buccopharyngeal anatomy, we dissected two tadpoles in Gosner stages 30 and 31 according to Wassersug (1976) and, after inspection using a stereoscopic microscope, we submitted them to the protocol of Alcalde and Blotto (2006) for scanning electron microscopy (SEM). Terminology of buccopharyngeal features follows Wassersug (1976, 1980).
Musculo-skeletal system
To study musculo-skeletal system, six tadpoles in Gosner stages 33 and 41 were processed according to the clearing and double staining protocol of Dingerkus and Uhler (1977); for two individuals, the procedure was interrupted after the alcian blue step, and specimens were manually dissected for inspection of cranial muscles. We employed lugol solution to aid in the identification of muscles. The protocol was carried out until the end for the remaining individuals aiming to study the larval cranium. Terminology for the musculo-skeletal elements is that of Haas (1995, 2001, 2003).
Phylogenetic relationships and character optimization
Besides Frostius pernambucensis, we examined tadpoles of other representatives of several genera of Neotropical bufonids (see complete list of examined material in the Appendix 1) to investigate character evolution within the family; our sampling was complemented with information available in the literature. We based our taxon sampling in the phylogenetic hypothesis of Jetz and Pyron (2018) which has a dense taxonomic sampling, although F. pernambucensis is not included in it. Besides Cannatella`s (1986) hypothesis, F. pernambucensis were never included in another phylogenetic hypothesis and the monophyly of the genus have never been tested. Nevertheless, based on adult morphology and behavior, we assumed the monophyly of the genus and the sister relationship of F. pernambucensis and F. erythrophthalmus—diagnostic characters, such as the iris coloration have been demonstrated polymorphic within F. erythrophthalmus, and all call parameters evaluated so far are overlapping between the two species (Juncá et al. 2012) rendering both species phenotypically very similar—for comparisons and optimizations purposes. We do not claim that we have an exhaustive sampling of bufonids, for that would be beyond of the scope of this paper; we recognize the extensive variation of bufonids, and we claim for further studies with deeper taxon and character sampling. Our analysis represents an initial step toward a better understanding of the evolution of bufonid larvae. Finally, the lack of data for several basal lineages, such as Oreophrynella, prevent an unambiguous optimization of some characters.
We delimited 25 transformation series (Hennig 1960; Grant and Kluge 2004) to describe the variation observed (Appendix 2), focusing on character-states of Frostius pernambucensis. The character matrix was constructed in Mesquite V. 3.51 (Maddison & Maddison 2018). These characters were optimized using parsimony as a criterion (Fitch 1971) into the topology of Jetz and Pyron (2018) using T.N.T.v. 1.5 (Goloboff & Catalano 2016). This tree was pruned to reflect the relationships among taxa for which characters were scored, focusing on the generic-level relationships; notwithstanding, we kept taxa for which no data are available to highlight the parts of the bufonids tree of life that require more studies on larval morphology. Most of the characters in our matrix are invariable within each genus, and most of the unique states are restricted to Frostius pernambucensis. We complemented our observations with information available in the literature. Finally, it is worthy to stress that our analysis is exploratory intended to identify some interesting states and major evolutionary patters in Frostius evolution; future studies including a more detail taxonomic sampling and phylogenetic analysis are required to test our propositions.
Natural history
We provide comments on the natural history and breeding behavior of Frostius pernambucensis based on field observations. Additionally, we briefly comment on the egg clutch based on field and laboratory observations (MHNUFAL 17359; collected at Reserva Particular do Patrimônio Natural Eco Fazenda Morim).
Results
External morphology
Total length 17.21 ± 1.06 mm (15.65–18.33 mm; n = 5; stage 38; Fig. 2). Body ovoid in dorsal and ventral views (MBW/BL = 0.52–0.58), depressed and globular in lateral view (MBH/MBW = 0.76–0.86). Ventral contour of body sloping, slightly concave in peribranchial region and convex in abdominal region. Body length about 1/4 of total length (BL/TL = 0.26–0.32); maximum body width on middle of body and maximum body height on its end portion. Snout oval in dorsal view and truncate in lateral view, slightly sloping. Eyes located dorsally and directed laterally, representing nearly 32% of intraorbital distance (ED/IoD = 0.30–0.37) and 24% of maximum body width (ED/MBW = 0.23–0.27). Nostrils dorsal and directed frontolaterally, with external opening reniform (Fig. 2F), and a small cutaneous projection (apophysis) on its inner margin; each nostril represents about 14% of intranarial distance (ND/InD = 0.14), 4% of maximum body width (ND/MBW = 0.04) and located closer the eyes than the tip of snout. Oral disc equivalent to 36% of maximum body width (ODW/MBW = 0.34–0.38), ventral, arched-shape, lips modified in a thick dermal fold laterally interrupted (Fig. 2D). Marginal papillae present, short and blump; submarginal papillae absent. Labial tooth row formula (LTRF) 1/1; A1 arched, P1 U-shaped, and shorter in length than A1; large and sparse labial teeth, 24–26 labial teeth in A1 and 8–10 in P1. Labial teeth composed of a basal sheath and convex head with poorly developed cusps (Fig. 3). Jaw sheaths with only the terminal portion pigmented; upper jaw sheath arc-shaped and finely serrated, lower jaw sheath open U-shaped and finely serrated, more robust than the upper jaw. Spiracle sinistral (Fig. 2E), located below the body midline, opening on middle third of body (SPD/SSD = 0.63–0.71), directed posterodorsally, forming an angle of approximately 45° with the longitudinal body axis, visible from ventral view. Inner wall free from body and external wall slightly shorter than inner wall. Vent tube with medial opening (Fig. 2G and H), longer than wide, with both walls attached directly to the ventral fin, except the tip, which is free. Tail length approximately 72% of total length (TaL/TL = 67.92–74.09), higher at the middle of tail; maximum height corresponding to half the height of body (MBH/MTH = 0.74–0.85). Tail tip acute. Maximum tail musculature height less than 1/3 of the maximum height of tail (TMH/MTH = 0.33–0.37), becoming progressively thinner posteriorly and extending to tail tip. Myosepta partially visible from half of its length. Dorsal fin height 32% of tail height (DFH/MTH = 0.30–0.37) and slightly higher than ventral fin (VFH/DFH = 0.30–0.33). Dorsal fin beginning at the end of body, with contour parallel to caudal musculature in almost all its length, slightly arched in its second half, maximum higher at the beginning of the last third. Ventral fin beginning at the base of tail, with origin concealed by vent tube and contour parallel to the caudal musculature; maximum height at first third of tail. Measurements are presented in Table 1.
Tadpole of Frostius pernambucensis, stage 36 (MHNUFAL 16196), in A lateral, B dorsal, and C ventral views. D Detail of the oral disc. E Detail of the spiracle. F Detail of the narial aperture. Detail of the vent tube in G lateral (right side) and H ventral views. Details of the eggs of F. pernambucensis (I). Lateral, dorsal, and ventral views of tadpoles of F. pernambucensis in stages (J) 28, (K) 32 and (L) 42 (MHNUFAL 16196). M Tadpole of F. pernambucensis, stage 37 (MHNUFAL 16196), in life. Scale bars = 3 mm (A–C, I–L), and 0.3 mm (D–H)
Coloration
In life, body Drab-Gray (256), covered by numerous Fawn Color (258) dots (Fig. 2L). Due to its translucency, in dorsal view, it is possible to observe Tawny Olive (17) color of the calf on lateral margins of the second half of body, as well as Vandyke Brown (281) color in inner part of the eyeballs. In ventral view, Fawn Color (258) points restricted to lateroventral surfaces, becoming less evident in the medial portion of belly. Belly Pale Buff (1), and evident calf in its posterior portion. In lateral view, posterior portion of body darker, Fawn Color (258). Translucent fins color Light Sky Blue (191) and caudal musculature opaque, colors similar to fins. Myosepta partially visible. In preservative, color pattern is maintained, background becomes Pale Horn Color (11) and dots distributed on the body become Dark Brownish Olive (127) (Fig. 2A–C).
Variation
Tadpoles show little variation in relation to morphological characteristics analyzed. However, when compared to more initial stages of development, a progressive change in body's coloration in life is observed, ranging from a clear translucent tone in Sulphur Yellow (80) and very evident calf in initial stages to a dark tone in Dark Lavender (203) and calf almost imperceptible in metamorphic stages. The eyes also develop becoming larger as development progresses. It is worth noting that tadpoles at stage 26 already have a total length similar to those in final stages of development (~ 16 mm); all growth is post-metamorphic (Fig. 2I–K; Table 1).
Buccopharyngeal cavity (Fig. 4)
Buccal roof
Buccal roof elliptical (Fig. 4A). Obtuse, medial, short papilla present in the prenarial arena (Fig. 4C). Internal nares elliptical, sagittally oriented in an angle of 60°; posterior valve free (Fig. 4D). Pre and postnarial papillae absent. Median ridge absent. Buccal roof arena absent. Buccal roof devoid of pustulation and papillae. Dorsal velum poorly developed, V-shaped, medially interrupted.
Buccopharyngeal cavity of a tadpole of Frostius pernambucensis (EI 7253) at stage 30. A Buccal floor, B buccal roof, and C details of the prenarial arena papilla, D internal nares, E infralabial papillae, and F lingual papillae. BP buccal pocket, BRP buccal roof papilla, IL infralabial papillae, IN internal nares, LB lingual bud, LJS lower jaw sheath, LP lingual papillae, PNAP prenarial, UJS upper jaw sheath. Scale bars = 100 µc (A, B, E, F), and 20 µm (C, D)
Buccal floor
Buccal floor triangular (Fig. 4B). Single pair of short, blunt, infralabial papillae (Fig. 4E). Lingual bud rounded; three short, round, lingual papillae (Fig. 4F). Buccal floor arena absent; single buccal floor papillae present above the buccal pocket line. Prepocket pustulation and papillae present. Buccal pockets deep, perforated, oblique, slit-shaped. Ventral velum present, reduced, inconspicuous; spicular support inconspicuous; secretory pits absent; secretory ridges absent; marginal projections absent; medial notch absent. Glottis fully exposed. Branchial basket reduced, semi-circular; filter cavities reduced; filter plates absent.
Musculature
We observed 22 muscles in the larvae of Frostius pernambucensis (Table 2; Fig. 5). Some muscles typically observed in other tadpoles, such as levator mandibulae lateralis (Haas 2003), were not observed; we stress the lack of this muscle is likely to be an observational problem rather than the loss of the muscle in the species. We suggest that future studies incorporating other techniques, such as histology and immunohistochemistry, could aid in the detection of these small muscles. Nevertheless, it is interesting noting the shift of some muscles, such as the levator arcuum branchialium IV, that inserts on the ceratobranchial III, the subarcualis rectus I that has two slips inserting on the processus branchialis II, and the suspensioangularis originating ventrally in the palatoquadrate.
Larval musculature of Frostius pernambucensis at stage 33 (MHNUFAL 16196). A–B Ventral view and C–D lateral view. GH geniohyoideus, IH interhyoideus, IM intermandibularis, LMLP levator mandibulae longus profundus, OH orbitohyoideus, RA rectus abdominis, RC rectus cervicis, SA suspensorioangularis, SH suspensoriohyoideus, SAR I subarcualis recuts I, SAR II–IV subarcualis rectus II–IV, SO subarcualis obliquus, TP tympanopharyngeus. Scale bars = 1.0 mm
Larval cranium
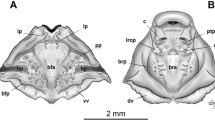
Neurocranium triangular; greatest width at otic capsule level (Fig. 6). Suprarostral cartilage (Fig. 6C) formed by two elements, suprarostral alae and corpus completely fused, although the line of fusion seems to be recognizable; dorsal posterior process of the suprarostral ala present, suprarostral corpora fused distally. Suprarostral corpus fused to the cornua trabeculae. Ethmoidal region short; cornua trabeculae short, parallel to each other and fused terminally with the suprarostral corpus. Basicranial fenestra open. Frontoparietal fenestra large, rectangular. Orbital cartilage (Fig. 6d) poorly developed, not reaching the otic capsule (foramen prooticum open). Capsula auditiva robust, large, rhomboidal in dorsal view, representing about 40% of chondrocranium length. Palatoquadrate thin in lateral view (Fig. 6C), attached to neurocranium through a wide anterior commissura quadratocranialis and an almost perpendicular processus ascendens posteriorly; processus ascendens with a high (sensu Haas 2003) attachment. Processus muscularis quadrati triangular, poorly developed, and curved dorsomedially. Commissura quadratoorbitalis absent. In the lower jaw (Fig. 6E), Meckel’s cartilage sigmoid, transversely oriented, almost perpendicular to the chondrocranium longitudinal axis. Infrarostral cartilages rectangular in frontal view, curved, joined at the symphysis.
Larval cranial skeleton of Frostius pernambucensis at stage 41 (MHNUFAL 16196). A Dorsal view; B ventral view; C detail of the suprarostral cartilage; D lateral view; E detail of the lower jaw; F hyobranchial apparatus. CA capsula auditiva, CB ceratobranchial, CI cartilago infrarostralis, CM cartilago Meckeli, CT cornua trabeculae, FO foramen opticum, FOC foramen oculomotorium, FPO foramen prooticum, PAH processus anterior hyalis, PAQ pars articularis quadrati, PAS processus ascendens, PHB planum hypobranchiale, PM processus muscularis, PRU pars reuniens, SA suprarostral ala, SC suprarostral corpus. Scale bars = 0.5 mm
Ceratohyals (Fig. 6F) short, flat, and subtriangular; anterior margin with poorly developed anterior and anterolateral processes, posterior processes triangular. Ceratohyals confluently joined by a chondrified pars reuniens. Basibranchial rectangular, with rounded processus urobranchialis present; basihyalis absent. Hypobranchial plates long, rectangular, free from each other. Branchial basket with four curved ceratobranchials, lacking lateral projections; ceratobranchials I, II, and III attached to the hypobranchial plate; ceratobranchial IV reduced, free. Ceratobranchial I with a triangular anterior processus branchialis. Spicules absent.
Natural history notes
Spawns and tadpoles of Frostius pernambucensis were observed both in hollow trees (n = 7) and in bromeliads (n = 2) containing accumulated rainwater. All reproductive sites were inside the forest, at least 200 m from the nearest edge, varying between 60 and 150 cm in height (Fig. 7). Males of F. pernambucensis were observed close to or inside the reproductive sites in almost all visits, many times in vocalization activity (during the night or late afternoon and on rainy days) and showed parental care behaviors during the collection of larvae.
Breeding sites of Frostius pernambucensis in Estação Ecológica de Murici, municipality of Murici, Alagoas state. A–C Different spawning developing in the same tree hollow with water accumulated at about 150 cm in height. D–E Spawn developing in the central cup of an epiphytic bromeliad with water accumulated at about 100 cm in height (see a video in Supplementary Material 1). F–G Spawn developing in tree hollow with water accumulated at about 60 cm in height
All clutches found had a large number of eggs and/or tadpoles (~ 20 to 50 individuals), even in reproductive sites with reduced space, such as in bromeliads (Fig. 7D and E; see a video of the density of tadpoles in the same bromeliad in Supplementary Material 1). The clutches (Fig. 2I) consist of gelatinous string of yellowed eggs; the entire string measured 4.4 cm on average (4.3–4.5 ± 0.08; n = 5), accommodating eggs of 2.0 mm (1.8–2.3 ± 0.13; n = 10).
Some clutches had tadpoles in early (newly hatched) and advanced (above stage 38) stages of development, indicating that the same reproductive site was used simultaneously more than once. The reuse of the same reproductive site consecutive times during the reproductive period was observed, with different spawns observed in June, July, and August 2021. During a visit to a tree hollow whose tadpoles were in an advanced stage of development, the behavior of cannibalism was observed, where some individuals feeding on two dead tadpoles, which already had most of the tail devoured.
Discussion
Larval morphology and the systematics of bufonids
More than 65% of known genera of bufonids have information on larval morphology, showing high phenotypic and ecological diversity (e.g., Duellman and Lynch 1969; Lamotte and Xavier 1972; Müller et al. 2005; Haas et al. 2009; Meegaskumbura et al. 2015; Viertel and Channing 2017; Müller 2019; Romero-Carvajal et al. 2023), with many larval characters presenting interesting evolutionary history and phylogenetic significance (Channing 1978; Haas 2003; Frost et al. 2006; Hirschfeld et al. 2012). Very few larval external features of Frostius pernambucensis support the notion that the species is part of the family Bufonidae, such as the eyes placed dorsally, the medial vent tube, and the sinistral spiracle (located in general below the medial line). On the other hand, many features are unique to F. pernambucensis tadpoles, probably related to its phytotelmata habits and endotrophic development (discussed below), suggesting putative synapomorphies—although the lack of information for its putative sister species, F. erythrophthalmus, and some other lineages of bufonids, precludes unambiguous optimizations.
The majority of bufonids tadpoles are characterized by a tail of moderated length that ends in a rounded tip. Moreover, the oral disc is in general emarginated, with marginal papillae absents dorsally and ventrally (putative synapomorphy of the family; Haas 2003) and a standard LTRF 2/3 (e.g., McDiarmid and Altig 1990; Inchaustegui et al. 2014; Vera Candioti et al. 2020). The long tail (representing ¾ of the total length) and the acute tail tip are two features that seems singular to F. pernambucensis, standing out from most other bufonids. However, the simplified aspect of the oral disc is the one that deserve the most attention. The marginal papillae are reduced, the lips are modified in a thick dermal fold interrupted laterally, the labial teeth are reduced to two rows (one anterior and one posterior) with few and sparce teeth, and jaw sheaths are almost inconspicuous with only the terminal portion pigmented. Among bufonids, endotrophic larvae are rare, also being reported for Blythophryne, Pelophryne, and Altiphrynoides (Grandison 1978; Müller 2019). Endotrophy evolved independently in those taxa, and some character-states associated with lack of feeding, such as the reduction of mouthparts, are convergent in those lineages.
The oral discs of these taxa present unique character-states; Blythophryne beryet has marginal papillae (with dorsal gap) and a well-developed jaw sheath, but no labial teeth (LTRF 0/0) (Chandramouli et al. 2016); while Pelophryne spp. have the oral disc with no observable marginal papillae and a LTRF 1/0 (Inger 1960; Malkmus et al. 2002; Leong and Teo 2009). The LTRF 1/1 and the presence of a modified lip in a thick dermal fold are autapomorphic conditions of F. pernambucensis and could represent a synapomorphy of Frostius, pending data on F. erythrophthalmus.
Data on buccopharyngeal cavity are available for several lineages of bufonids: Anaxyrus (Wassersug and Rosenberg 1979); Altiphrynoides (Müller 2019); Ansonia (Inger 1985); Bufo (Viertel 1982); Ingerophrynus (Inger 1985); Melanophryniscus (Baldo et al. 2014); Osornophryne (Romero-Carvajal et al. 2023); Poyntonophrynus (Lambris 1994); Rentapia (Inger 1985); Rhinella (e.g., Vera Candioti 2007; Oliveira et al. 2013); Schismaderma (Lambris 1994; Viertel and Channing 2017); Sclerophrys (Lambris 1994); and Vandijkophrynus (Lambris 1994). Although some features may be missing in some taxa, the almost complete absence of elements in the buccal roof and buccal floor is unique to the tadpoles of F. penambucensis. However, the lack of information for several lineages of bufonids (e.g., Oreophrynella) prevents an unambiguous optimization of these characters. We address, though, some interesting patterns that must be elucidated in the future.
Frostius pernambucensis has a single pair of infralabial papillae, the same as other bufonids for which the buccopharyngeal cavity is known (e.g., Oliveira et al. 2013; Müller 2019; Vera Candioti et al. 2020; this study), including the most basal genus of the family, Melanophryniscus (Baldo et al. 2014), contrasting with the four papillae (two pairs) observed in Bufonidae`s sister taxa Odontophrynidae (e.g., Nascimento et al. 2013; Dias et al. 2014, 2019). Other closely related lineages, such as centrolenids or leptodactylids (mostly), also present two pairs of infralabial papilla (e.g., Wassersug and Heyer 1988; Vera Candioti et al. 2007; Rada et al. 2019; Dias et al. 2020; Nascimento et al. 2020). Thus, we suggest that the presence of a single pair of infralabial papillae is a putative synapomorphy of Bufonidae.
We observed three, rounded, poorly developed lingual papillae in F. pernambucensis. Most bufonids present four lingual papillae with few exceptions: lingual papillae are absent in members of the Rinella veraguensis species group (Cadle and Altig 1991; Aguayo et al. 2009), and in Ansonia tadpoles (Inger 1985), R. rumboli may have 3 or 4 lingual papillae (Haad et al. 2014), and Ingerophrynus divergens present a single pair (Inger 1985). Three lingual papillae seem to be a unique feature of Frostius, although the occurrence of polymorphic condition in other bufonids, such as R. rumboli, cast a shadow in that interpretation; further specimens of Frostius need to be examined regarding their buccopharyngeal cavity to determine the occurrence or not of polymorphism in this character. It is worthy to note that, despite the number of papillae, the reduction in its size has never been reported in any other bufonid as far as we know.
The reduction in the number of buccal floor and roof papillae and pustulation is another characteristic of Frostius pernambucensis—single pair of buccal floor arena papillae and no pustulation at all—in comparison with other bufonids. Buccal roof papillae are absent in the larvae of Ansonia (Inger 1985), but all other bufonids with their buccopharyngeal cavity described present some papillae and pustulations on the buccal roof and floor. Also, postnarial papillae are missing in Frostius. We thus suggest that the absence of pustulations on the roof and floor, as well as the absence of papillae in the buccal roof and postnarial papillae could represent synapomorphies for the genus Frostius. Other elements are missing the buccopharyngeal cavity of Frostius tadpoles: median ridge, lateral ridge papillae, secretory ridges and secretory pores, and filter plates, that should be better explored in other bufonid genera, but could represent putative synapomorphies for the genus.
The musculo-skeletal system of Frostius pernambucensis is also highly modified, with interesting evolutionary implications. Haas (2003) suggested some larval synapomorphies derived from the muscles and cartilages for the family Bufonidae: (1) the absence of m. interhyoideus posterior; (2) the absence of the diaphragmatopraecordialis; (3) a slip of the subarcualis rectus II–IV reaches far laterally into interbranchial septum IV; and 4) the absence of processus anterolateralis. Frostius pernambucensis is in accordance with three of these synapomorphies, but differs regarding the subarcualis rectus II–IV, which originates in the ceratobranchial III and reaches the ceratobranchial I, both conditions are putative synapomorphies for the genus.
Many bufonids have the m. subarcualis rectus I divided into two slips inserting on the ceratobranchials II and III (e.g., Haas 2003; Vera Candioti 2007). In Frostius pernambucensis, the two slips are also present, but both are inserted in the ceratobranchial III; the changing in the pattern of insertion of the dorsal slip is a putative synapomorphy of Frostius. Also, the insertion of the levator arcuum branchialium IV and that of the tympanopharyngeus are modified in F. pernambucensis; in this species, all the mentioned muscles are inserting on the ceratobranchial III, in contrast with all other bufonids, in which these muscles insert on the ceratobranchial IV.
Regarding the larval cranium, Frostius pernambucensis also has several interesting transformations. Suprarostral corpus and alae completely fused it is not commonly observed among bufonids, but it was reported for the gastromyzophorous larvae of Atelopus (Lavilla and de Sá 2001). Amazophrynella, Dendrophryniscus, and Melanophryniscus do not present such fusion (Baldo et al. 2014; P.H.D. pers.obs.), but the lack of data for other basal lineages creates a difficulty in optimizing this character state. Notwithstanding, the fusion on the suprarostral with the cornua trabeculae had never been reported in bufonids—Gastrophryne carolinensis larvae have these cartilages fused (Trueb et al. 2011), but this is not closely related species—which represents an additional putative synapomorphy of Frostius.
The commissura quadratoorbitalis is present in the majority of bufonids (Haas 2003), but gives its presence in other closely related taxa, such as Odontophrynidae (Dias et al. 2019); it could represent a synapomorphy of a more inclusive clade. Such commissura was lost in F. pernambucensis, representing another putative synapomorphy for the genus.
Finally, the ceratobranchials II and III attached to the hypobranchial plates, the absence of commissura terminalis uniting the ceratobranchial, and the free ceratobranchial IV are also putative synapomorphies of the genus, given that these conditions are not observed in other members of the family (e.g., Haas 2003; Aguayo et al. 2009; Haad et al. 2014).
Living in phytotelmata and the evolution of endotrophy
Phytotelmata, such as bromeliads tanks, bamboo stumps, tree holes, nut shells, and palm leaves, can be defined as water filled plants or parts of plants (Thienemann 1934). This habitat can be drastically different from other water bodies in its physicochemical properties, presenting low dissolved oxygen, low pH, and elevated viscosity (Laessle 1961; Maguire. 1971; Richardson 1999). Another characteristic of phytotelmata is that the food chain and the primary productivity are very particular (Richardson et al. 2000; Kitching 2001) with reduced availability of food resources, and thus, organisms inhabiting those habitats may face distinguished selective pressures. Frogs have colonized phytotelmata several times (Lehtinen et al. 2004) and tadpoles developing in that environment have often evolved differentiated trophic strategies; although many phytotelm dwellers are detritivorous and filterers (e.g., Caldwell 1993; Dias and Pie 2021), several species are predators (carnivory), oophagous, or endotrophic (Lannoo et al. 1987; Lehtinen et al. 2004). Within bufonids, phytotelm usage for development has been reported in several taxa (e.g., Caldwell 1993; Langone et al. 2008; Malkmus and Dehling 2008), and the tadpoles of some species inhabiting these plants are endotrophic (e.g., Leong and Teo 2009; Chandramouli et al. 2016).
Most of unique character-states in Frostius pernambucensis are related to the reduction or loss of elements in oral disc, buccopharyngeal cavity, musculature, and larval cranium. Such pattern can be directly related to the endotrophic development of these tadpoles. Endotrophic tadpoles usually lack mouthparts, pigmentation, tail fins, and spiracle (e.g., Kaiser and Altig 1994; Caldwell and Lima 2003; Randrianiaina et al. 2011; Etter et al. 2021), although a variety of combination of traits losses can be observed in different lineages (Altig and Johnston 1989).
The buccopharyngeal cavity of several endotrophic larvae follow similar pattern, with the reduction or loss of features. Wassersug and Duellman (1984) investigated the phenotypic variation in buccopharyngeal cavity of several hemiphractid tadpoles. Their findings regarding the endotrophic forms are similar to our results with Frostius pernambucensis, including the loss or reduction of several papillae and other buccal features.
Similarly, the musculo-skeletal system is quite modified in endotrophic forms. We reported some differences regarding the pattern of origin and insertion of some muscles (e.g., levator arcuum branchialium), although the general pattern of origin and insertion of muscles in quite similar to that of exotrophic tadpoles. This is also true for other endotrophic tadpoles for which larval muscles are known—Eupsophus emiliopugini (Vera Candioti et al. 2011a, b) and Fritziana goeldi (Haas 1996).
Larval cranium morphology data are available for few endotrophic tadpoles: Cycloramphus stejnegeri (Lavilla 1991), Eupsophus calcaratus (Vera Candioti et al. 2005), E. emiliopugini (Vera Candioti et al. 2011a, b), E. nahuelbutensis (Nuñez and Úbeda 2009), E. queulensis (Cárdenas-Rojas et al. 2007), Rhinoderma darwinii (Lavilla 1987), and Fritziana goeldi (Haas 1996). Some character-states present in most of these larvae, as well as in Frostius pernambucensis, are the short processus muscularis quadrati, large capsula auditiva, short cornua trabecula, tall orbital cartilage, and reduced hyobranchial apparatus. With exception of the enlargement of capsula auditiva, these character-states can be directly correlated with the lack of feeding activity in these larvae.
Two no exclusive mechanisms have been evoked to explain the evolution of endotrophy: (1) the loss of some specific tadpole characters and (2) the acceleration in the development of some adult characters (Elinson 2001). Empirical evidence seems to support these ideas; data suggest that endotrophy is associated with the remodeling and/or loss of cartilaginous elements typical of tadpoles, as the suprarostral cartilages and palatoquadrate, and with changes in the onset of some skeletal elements (Hanken et al. 1992; Yeh 2002; Kerney et al. 2007). The fusion between the suprarostral and cornua trabeculae provides additional evidence to the idea of truncation of development. The suprarostral cartilage is likely evolved by the addition of an articulation in the existing trabecular cartilages (Sevensson and Haas 2005; Lukas and Olsson 2018) during development; the fact that these cartilages are not completely separated could indicate that tadpole`s development was truncated.
Changes in development is also valid for buccopharyngeal cavity; Wassersug and Duellman (1984) examined the ontogeny of buccopharyngeal cavity in the exotrophic larvae of Gastrotheca riobambae and observed that early stage embryos (Gosner 23) lacked most of buccal features and resembled the cavity of endotrophic forms and concluded (Wassersug and Duellman 1984:35) that endotrophic forms may have arisen by a simple truncation or acceleration of the “tadpole developmental program”. As far as we know, there is no information on the buccopharyngeal cavity of bufonids in early developmental stages (24-), but the external morphology of F. pernambucensis resembles that of the embryos of other bufonids, with poorly developed oral discs and mouthparts and poorly pigmented bodies (Vera Candioti et al. 2016).
The real processes involved in the evolution of endotrophy are still poorly understood. Altig and Crother (2006) hypothesized that endotrophic forms evolved from developmental reppartening via changes in some gene family responsible for the development of tadpole-specific traits (see also Altig 2006). Although there are no molecular and developmental studies addressing this issue, the fact that the loss of exotrophic tadpoles and the evolution of endotrophic forms occurred independently and repeatedly in some lineages (besides bufonids), such as aromobatids (e.g., Vacher et al. 2017) and hemiphractids (e.g., Castroviejo Fischer et al. 2015), suggests that some regulatory program can be altered relatively easily. Further studies are necessary to test these hypotheses.
Finally, endotrophy also had an impact in the ecological interactions and space use in Frostius pernambucenis larvae. We observed several individuals in the same phytotelm (up to 50 tadpoles at different developmental stages). Usually, phytotelm breeders tend to lay few eggs per site; for example, in dart-poison frogs (Dendrobatidae) the number of eggs in the clutches and tadpoles deposited per site tend to be small (Caldwell and Araújo 1998; Lehtinen et al 2004; Duarte-Marin et al. 2020). Large number of tadpoles inhabiting the same phytotelm seems to be correlated with oophagy (Schiesari et al. 2003), and we suggest that the large number of F. pernambucenis tadpoles found in a single site could be related to endotrophy, giving that feeding resources would not be a limiting factor for special usage. Further studies are necessary to test this hypothesis.
Remarks and conclusions
The endotrophic tadpoles of Frostius pernambucenis are highly modified in comparison with exotrophic larvae of other bufonids, leading us to propose several novel synapomorphies for the genus. Notwithstanding, data are scarce for many lineages of bufonids (especially for early divergent clades), and a more comprehensive study will be necessary to test our hypotheses.
The colonization of phytotelma, an environment relatively poor of food resources, could have created a selective pression on the development of F. pernambucenis, favoring the evolution of endotrophy, although further evidence is necessary to test this hypothesis. The evolution of non-feeding larvae is associated with the loss and/or remodeling of several elements of the anatomy of these tadpoles. This fact suggests that F. pernambucensis might be an excellent model for future evo-devo studies.
Data availability
All relevant data are within the manuscript.
References
Aguayo R, Lavilla EO, Vera Candioti MF, Camacho T (2009) Living in fast-flowing water: morphology of the gastromyzophorous tadpole of the bufonid Rhinella quechua (R. veraguensis group). J Morphol 270:1431–1442
Alcalde L, Blotto B (2006) Chondrocranium, cranial muscles and buccopharyngeal morphology on tadpoles of the controversial leptodactylid frog Limnomedusa macroglossa (Anura: Leptodactylidae). Amphibia-Reptilia 27:241–253
Altig R (2006) Tadpoles evolved and frogs are the default. Herpetologica 62:1–10
Altig R, Crother BI (2006) The evolution of three deviations from the biphasic anuran life cycle: alternatives to selection. Herpetol Rev 37:321–325
Altig R, Johnston GF (1989) Guilds of anuran larvae: relationships among developmental modes, morphologies, and habits. Herpetol Monogr 3:81–109. https://doi.org/10.2307/1466987
Altig R, McDiarmid RW (1999) Tadpoles: The Biology of Anuran Larvae. The University of Chicago Press, Chicago
Baldo D, Vera Candioti MF, Haad MB, Kolenc F, Borteiro C, Pereyra MO, Zank C, Colombo P, Bornschein MR, Sisa FN, Brusquetti F, Conte CE, Nogueira-Costa P, Almeida-Santos P, Pie MR (2014) Comparative morphology of pond, stream and phytotelm-dwelling tadpoles of the South American Redbelly Toads (Anura: Bufonidae: Melanophryniscus). Biol J Linn Soc 112:417–441. https://doi.org/10.1111/bij.12296
Barbosa V, Pereira E, Maranhão E (2017) Anfíbios da Estação Ecológica de Caetés Paulista, Pernambuco-atualização da lista de espécies. Rev Ciên Amb 11:39–49. https://doi.org/10.18316/rca.v11i2.3003
Bokermann WCA (1962) Una nueva especies de Atelopus del nordeste de Brasil (Amphibia, Salientia, Brachycephalidae). Neotropica 8:42–44
Cadle JE, Altig R (1991) Two lotic tadpoles from the Andes of Southern Peru: Hyla armata and Bufo veraguensis, with notes on the call of Hyla armata (Amphibia: Anura: Hylidae and Bufonidae). Stud Neotrop Fauna 1:45–53
Caldwell J (1993) Brazil nut fruit capsules as phytotelmata: interactions among anuran and insect larvae. Can J Zool 71:1193–1201
Caldwell JP, de Araújo MC (1998) Cannibalistic interactions resulting from indiscriminate predatory behavior in tadpoles of poison frogs (Anura: Dendrobatidae). Biotropica 30:92–103
Caldwell JP, Lima AP (2003) A new amazonian species of Colostethus (Anura: Dendrobatidae) with a nidicolous tadpole. Herpetologica 59:219–234
Cannatella DC (1986) A new genus of bufonid (Anura) from South America, and phylogenetic relationships of the Neotropical genera. Herpetologica 42:197–205
Cárdenas-Rojas DR, Veloso A, de Sá EO (2007) The tadpole of Eupsophus queulensis (Anura, Cycloramphidae). Alytes 25:45–54
Castroviejo-Fischer S, Padia JM, de La Riva I, Pombal JP Jr, Silva HR, Rojas-Runjaic FJM, Medina-Méndez E, Frost DR (2015) Phylogenetic systematics of egg-brooding frogs (Anura: Hemiphractidae) and the evolution of direct development. Zootaxa 4004:1–75
Chandramouli SR, Vasudevan K, Harikrishnan S, Dutta SK, Janani SJ, Sharma R, Das I, Aggarwal RK (2016) A new genus and species of arboreal toad with phytotelmonous larvae, from the Andaman Islands, India (Lissamphibia, Anura, Bufonidae). ZooKeys 555:57–90. https://doi.org/10.3897/zookeys.555.6522
Channing A (1978) A new bufonid genus (Amphibia: Anura) from Rhodesia. Herpetologica 34:394–397
Channing A, Rödel MO, Channing J (2012) Tadpoles of Africa: the biology and identification of all known tadpoles in sub-Saharan Africa. Edition Chimaira, Frankfurt
Chou WH, Lin JY (1997) Description of a new species, Rana multidenticulata (Anura: Ranidae), from Taiwan. Zool Stud Taipei 36:222–229
Cruz CD, Peixoto OL (1982) Sobre a biologia de Atelopus pernambucensis Bokermann, 1962 (Amphibia, Anura, Bufonidae). Rev Bras Biol 42:627–629
Dias PHS (2020) The remarkable larval anatomy of Proceratophrys minuta Napoli, Cruz, Abreu and Del-Grande, 2011 (Amphibia: Anura: Odontophrynidae). J Morphol 281:1086–1097
Dias PHS, Pie MR (2021) Buccopharyngeal morphology of the tadpoles of Scinax v-signatus, with comments on larval characters of the S. perpusillus species group (Amphibia: Anura: Hylidae). Zootaxa 4964:195–200
Dias PHS, Carvalho-e-Silva AMPT, Carvalho-e-Silva SP (2014) The tadpole of Proceratophrys izecksohni (Amphibia: Anura: Odontophrynidae). Zool 31:181–194
Dias PGS, Araujo-Vieira K, Santos RF, Both C (2019) Review of the internal larval anatomy of the Proceratophrys bigibbosa species group (Anura: Odontophrynidae), with description of the tadpole of P. brauni Kwet and Faivovich, 2001. Copeia 107:417–429
Dias PHS, Anganoy-Criollo M, Rada M, Grant T (2020) Comparative larval buccopharyngeal morphology of two glass frog species (Anura: Centrolenidae: Vitreorana). Zool Anz 289:118–122
Dias PHS, Vera Candioti F, Sabbag AF, Colaço G, Silva HR, Haddad CFB, Carvalho-e-Silva AMPT, Grant T (2021) Life on the edge: Tadpoles of Cycloramphidae (Amphibia; Anura), anatomy, systematics, functional morphology, and comments on the evolution of semiterrestrial tadpoles. J Zoolog Syst Evol Res 59:1297–1321. https://doi.org/10.1038/s41559-018-0515-5
Dias PHS, Marcondes BC, Pezzuti TL, Vera Candioti F, Prodocime MM, Silva HR, Orrico VGD, Haas A (2023) The missing piece of the puzzle: larval morphology of Xenohyla truncata (Anura: Hylidae: Dendropsophini) and its implication to the evolution of Dendropsophini tadpoles. Zoomorphol 142:111–126
Dingerkus G, Uhler LD (1977) Enzyme clearing of alcian blue stained whole small vertebrates for demonstration of cartilage. Stain Technol 52:229–232
Duarte-Marín S, González-Acosta CC, Santos Dias PH, Arias-Álvarez GA, Vargas-Salinas F (2020) Advertisement call, tadpole morphology, and other natural history aspects of the threatened poison frog Andinobates daleswansoni (Dendrobatidae). J Nat Hist 54:3005–3030
Dubeux MJM, Nascimento FAC, Lima LR, Magalhães FM, Silva IRS, Gonçalves U, Almeida JPFA, Correia LL, Garda AA, Mesquita DO, Rossa-Feres DC, Mott T (2020a) Morphological characterization and taxonomic key of tadpoles (Amphibia: Anura) from the northern region of the Atlantic Forest. Biota Neotrop 20:1–24. https://doi.org/10.1590/1676-0611-bn-2018-0718
Dubeux MJM, Gonçalves U, Nascimento FAC, Mott T (2020b) Anuran amphibians of a protected area in the northern Atlantic Forest with comments on topotypic and endangered populations. Herpetol Notes 13:61–74
Duellman WE (1984) Taxonomy of Brazilian hylid frogs of the genus Gastrotheca. J Herpetol 18:302–312
Duellman WE, Lynch JD (1969) Descriptions of Atelopus tadpoles and their relevance to atelopodid classification. Herpetologica 25:231–240
Elinson RP (2001) Direct development: an alternative way to make a frog. Genesis 29:91–95
Etter L, Haas A, Lee CC, Min PY, Das I, Hertwig ST (2021) Out of the trap: A new phytothelm-breeding species of Philautus and an updated phylogeny of Bornean bush frogs (Anura: Rhacophoridae). J Zool Sys Evol Res 59:1064–1096
Fitch WM (1971) Toward defining the course of evolution: minimum change for a specific tree topology. Syst Biol 20:406–416
Freitas MA, Abegg AD, Silva TFS, Fonseca PM, Hamdan B, Filadelfo T (2019) Herpetofauna of Serra do Timbó, an Atlantic Forest remnant in the state of Bahia, northeastern Brazil. Herpetol Notes 12:245–260
Frost DR, Grant T, Faivovich J, Bain RH, Haas A, Haddad CFB, de Sá RO, Channing A, Wilkinson M, Donnellan SC, Raxworthy CJ, Campbell JA, Blotto BL, Moler PE, Drewes RC, Nussbaum RA, Lynch JD, Green DM, Wheeler WC (2006) The amphibian tree of life. Bull Am Mus Nat 297:1–370. https://doi.org/10.1206/0003-0090(2006)297[0001:TATOL]2.0.CO;2
Frost DR (2023) Amphibian Species of the World: An Online Reference. Version 6.1 (Date of access 16 Jan 2023). Electronic Database accessible at https://amphibiansoftheworld.amnh.org/index.php. American Museum of Natural History, New York, USA. https://doi.org/10.5531/db.vz.0001
Goloboff PA, Catalano SA (2016) TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 3:221–238
Gosner KL (1960) A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica 16:183–190
Grandison AG (1978) The occurrence of Nectophrynoides (Anura: Bufonidae) in Ethiopia. A new concept of the genus with a description of a new species. Monit Zool Ital 11:119–172. https://doi.org/10.1080/03749444.1978.10736579
Grant T, Kluge AG (2004) Transformation series as an ideographic character concept. Cladistics 20:23–31
Grosjean S (2005) The choice of external morphological characters and developmental stages for tadpole-based anuran taxonomy: a case study in Rana (Sylvirana) nigrovittata (Blyth, 1855) (Amphibia, Anura, Ranidae). Contrib Zool 74:61–76. https://doi.org/10.1163/18759866-0740102005
Haad MB, Vera Candioti F, Baldo D (2014) The stream tadpoles of Rhinella rumbolli (Anura: Bufonidae). Herpetologica 70:184–197
Haas A (1995) Cranial features of dendrobatid larvae (Amphibia: Anura: Dendrobatidae). J Morphol 224:241–264
Haas A (1996) Non-feeding and feeding tadpoles in hemiphractine frogs: larval head morphology, heterochrony, and systematics of Flectonotus goeldii (Amphibia: Anura: Hylidae). J Zool Sys Evol Res 34:163–171
Haas A (1997) The larval hyobranchial apparatus of discoglossoid frogs: its structure and bearing on the systematics of the Anura (Amphibia: Anura). J Zoolog Syst Evol Res 35:179–197
Haas A (2001) Mandibular arch musculature of anurans tadpoles, with comments on homologies of Amphibian jaw muscles. J Morphol 247:1–33. https://doi.org/10.1002/1097-4687(200101)247:1%3c1::AID-JMOR1000%3e3.0.CO;2-3
Haas A (2003) Phylogeny of frogs as inferred from primarily larval characters (Amphibia: Anura). Cladistics 19:23–89. https://doi.org/10.1111/j.1096-0031.2003.tb00405.x
Haas A, Wolter J, Hertwig ST, Das I (2009). Larval morphologies of three species of stream toads, genus Ansonia (Amphibia: Bufonidae) from East Malaysia (Borneo), with a key to known Bornean Ansonia tadpoles. Zootaxa 2302:21–18. https://doi.org/10.11646/zootaxa.2302.1.1
Hanken J, Klymkowsky MW, Summers CH, Seufert DW, Ingebrigstein N (1992) Cranial ontogeny in the direct developing frog, Eleutherodactylus coqui (Anura, Leptodactylidae), analyzed with whole-mount immunohistochemistry. J Morphol 211:95–118
Hennig W (1960) Die Dipteren-Fauna von Neuseeland als systematisches und tiergeographisches Problem. Beitr Entomol 10:221–329
Hirschfeld M, Barej MF, Loader SP, Roedel MO (2012) Description of two Werneria tadpoles from Cameroon (Amphibia: Anura: Bufonidae). Zootaxa 3172:65–68. https://doi.org/10.11646/zootaxa.3172.1.5
Incháustegui SJ, Ng K, Marte C, Díaz LM (2014) The Tadpoles of the Southern Crested Toad (Peltophryne guentheri: Anura: Bufonidae) from Hispaniola. Reptiles & Amphibians 21:125–129. https://doi.org/10.17161/randa.v21i4.14011
Inger RF (1960) Notes on toads of the genus Pelophryne. Fieldiana 39:415–418. https://doi.org/10.5962/bhl.title.3348
Inger RF (1985) Tadpoles of the forested regions of Borneo. Fieldiana 26:1–89
Jetz W, Pyron RA (2018) The interplay of past diversification and evolutionary isolation with present imperilment across the amphibian tree of life. Nat Ecol Evol 2:850–858
Juncá FA, Röhr DL, Lourenço-de-Moraes R, Santos FJ, Protázio AS, Mercês EA, Solé M (2012) Advertisement call of species of the genus Frostius Cannatella 1986 (Anura: Bufonidae). Acta Herpetol 7:189–201
Juncá FA, Borges CLS (2002) Fauna associada a bromélias terrícolas da Serra da Jibóia-BA. Sitientibus 2:73–81. https://doi.org/10.13102/scb8239
Kaiser H, Altig R (1994) The atypical tadpole of the dendrobatid frog, Colostethus chalcopis, from Martinique, French Antilles. J Herpetol 28:374–378
Kenny JS (1969) Feeding mechanisms in anuran larvae. J Zool 157:225–246
Kerney R, Meegaskumbura M, Manamendra-Arachchi K, Hanken J (2007) Cranial ontogeny in Philautus silus (Anura: Ranidae: Rhacophorinae) reveals few similarities with other direct-developing anurans. J Morphol 268:715–725
Kitching RL (2001) Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Ann Rev Entomol 46:729–760
Köhler G (2012) Color catalogue for field biologists. Herpeton Verlag, Offenbach
Laessle AM (1961) A micro-limnological study of Jamaican bromeliads. Ecology 42:499–517
Lambris AJL (1994) Laryngeal and buccopharyngeal morphology of some South African Bufonidae: new data sets for anuran taxonomy. Ann Natal Mus 35:261–307
Lamotte M, Xavier F (1972) Recherches sur le développement embryonnaire de Nectophrynoides occidentalis Angel, amphibien anoure vivipare. I. Les principaux traits morphologiques et biométriques du développement Ann Embryol. Morphogenese 5:315–340
Langone JA, Segalla MV, Bornschein M, de Sá RO (2008) A new reproductive mode in the genus Melanophryniscus Gallardo, 1961 (Anura: Bufonidae) with description of a new species from the state of Paraná, Brazil. South Amer J Herpetol 3:1–9
Lannoo MJ, Townsed DS, Wassersug RJ (1987) Larval life in the leaves: arboreal tadpoles types, with special attention to the morphology, ecology, and behavior of oophagous Osteopilus brunneus (Hylidae) larvae. Fieldiana 38:1–31
Lavilla EO (1987) La larva de Rhinoderma darwinii D. & B. (Anura: Rhinodermatidae). Acta Zool Lilloana 39:81–88
Lavilla EO (1991) Condrocráneo y esqueleto visceral en larvas de Cycloramphus stejnegeri (Leptodactylidae). Amphibia-Repitillia 12:33–38
Lavilla EO, de Sá R (2001) Chondrocranium and visceral skeleton of Atelopus tricolor and Atelophryniscus chrysophorus tadpoles (Anura, Bufonidae). Amphibia-Reptilia 22:167–177
Lavilla EO, Scrocchi GJ (1986) Morfometría larval de los géneros de Telmatobiinae (Anura: Leptodactylidae) de Argentina y Chile. Physis 44:39–43
Lehtinen RM, Lannoo MJ, Wassersug RJ (2004) Phytotelma-breeding anurans: past, present and future research. Misc Publ Mus Zool Univ Michigan 193:1–9
Leong TM, Teo SC (2009) Endotrophic tadpoles of the Saint Andrew’s cross toadlet, Pelophryne signata (Amphibia: Anura: Bufonidae) in Singapore. Nat Singapore 2:21–25
Liedtke HC, Wiens JJ, Gomez-Mestre I (2022) The evolution of reproductive modes and life cycles in amphibians. Nat Commun 13:7039
Lukas P, Olsson L (2018) Bapx1 is required for jaw joint development in amphibians. Evol Devel 20:192–206
Maddison WP, Maddison DR (2018). Mesquite: a modular system for evolutionary analysis. Version 3.4.[Computer software]. www.mesquiteproject.org
Maglia AM, Pugener LA, Trueb L (2001) Comparative development of anurans: using phylogeny to understand ontogeny. Am Zool 41:538–551
Maguire B Jr (1971) Phytotelmata: biota and community structure determination in plant-held waters. Ann Rev Ecol Sys 2:439–464
Malkmus R, Dehling JM (2008) Anuran amphibians of Borneo as phytotelm-breeders – a synopsis. Herpetozoa 20:165–172
Malkmus R, Manthey U, Vogel G, Hoffmann P, Kosuch J (2002) Amphibians and Reptiles of Mount Kinabalu (North Borneo). Gantner Verlag, Rugell, A.R.G
McDiarmid RW, Altig R (1990) Description of a bufonid and two hylid tadpoles from western Ecuador. Alytes 8:51–60
Meegaskumbura M, Senevirathne G, Wijayathilaka N, Jayawardena B, Bandara C, Manamendra-Arachchi K, Pethiyagoda R (2015) The Sri Lankan torrent toads (Bufonidae: Adenominae: Adenomus): species boundaries assessed using multiple criteria. Zootaxa 3911:245–261. https://doi.org/10.11646/zootaxa.3911.2.6
Montesinos R, Carvalho AL, Silva HRD, Anganoy-Criollo M, Dias PHDS (2022) The tadpole of Hylodes perere Silva & Benmaman 2008 (Anura: Hylodidae). Zootaxa 5219:388–396
Müller H (2019) Description of the tadpole of the critically endangered Ethiopian toad Altiphrynoides osgoodi (Amphibia: Anura: Bufonidae). J Herpetol 53:218–223
Müller H, Measey GJ, Malonza PK (2005) Tadpole of Bufo taitanus (Anura: Bufonidae) with notes on its systematic significance and life history. J Herpetol 39:138–141. https://doi.org/10.1670/0022-1511(2005)039[0138:TOBTAB]2.0.CO;2
Nascimento FAC, Mott T, Langone JA, Davis CA, de Sá RO (2013) The genus Odontophrynus (Anura: Odontophrynidae): a larval perspective. Zootaxa 3700:140–158
Nascimento FAC, de Sá RO, Garcia PCA (2020) Tadpole of the Amazonia frog Edalorhina perezi (Anura: Leptodactylidae) with description of oral internal and chondrocranial morphology. J Morphol 282:115–126. https://doi.org/10.1002/jmor.21286
Noble GK (1929) The adaptative modifications of the arboreal tadpoles of Hoplophryne and the torrent tadpoles of Staurois. Bull Amer Mus Nat Hist 58:291–334
Nuñez JJ, Úbeda CA (2009) The tadpole of Eupsophus nahuelbutensis (Anura: Neobatrachia): external morphology, chondrocranium, and comments on its natural history. Zootaxa 2126:58–68
Oliveira MSRR, Weber LN, Napoli MF (2013) Internal oral morphology in larvae of the genus Rhinella Fitzinger, 1826 (Amphibia, Anura, Bufonidae). Zootaxa 3745:501–523
Orton G (1953) The systematic of vertebrate larvae. Syst Zool 2:63–75
Pimenta BV, Caramaschi U (2007) New species of toad, genus Frostius Cannatella, 1986, from the Atlantic rain forest of Bahia, Brazil (Amphibia, Anura, Bufonidae). Zootaxa 1508:61–68. https://doi.org/10.11646/zootaxa.1508.1.3
Pramuk JB, Robertson T, Sites JW Jr, Noonan BP (2008) Around the world in 10 million years: biogeography of the nearly cosmopolitan true toads (Anura: Bufonidae). Glob Ecol Biogeogr 17:72–83. https://doi.org/10.1111/j.1466-8238.2007.00348.x
Pugener LA, Maglia AM, Trueb L (2003) Revisiting the contribution of larval characters to an analysis of phylogenetic relationships of basal anurans. Zool J Linn Soc 139:129–155
Rada M, Dias PHDS, Pérez-Gonzalez JL, Anganoy-Criollo M, Rueda-Solano LA, Pinto-E MA, Quintero LM, Vargas-Salinas F, Grant T (2019) The poverty of adult morphology: Bioacoustics, genetics, and internal tadpole morphology reveal a new species of glassfrog (Anura: Centrolenidae: Ikakogi) from the Sierra Nevada de Santa Marta. Colombia Plos One 14:e0215349
Randrianiaina RD, Wollenberg KC, Hiobiarilanto TR, Straus A, Glos J, Vences M (2011) Nidicolous rather than direct development in Malagasy frogs of the genus Gephyromantis. J Nat Hist 45:2871–2900
Richardson BA (1999) The bromeliad microcosm and the assessment of faunal diversity in a Neotropical Forest. Biotropica 31:321–336
Richardson BA, Rogers C, Richardson MJ (2000) Nutrients, diversity, and community structure of two phytotelm systems in a lower montane forest, Puerto Rico. Ecol Entomol 25:384–356
Romero-Carvajal A, Negrete L, Salazar-Nicholls MJ, Vizuete K, Debut A, Dias PH, Vera Candioti F (2023) Direct development or endotrophic tadpole? Morphological aspects of the early ontogeny of the plump toad Osornophryne occidentalis (Anura: Bufonidae). J Morphol 284:e21582
Schiesari L, Gordo M, Hödl W (2003) Treeholes as calling, breeding, and developmental sites for the Amazonian canopy frog, Phrynohyas resinifictrix (Hylidae). Copeia 2003:263–272
Sevensson ME, Haas A (2005) Evolutionary innovation in the vertebrate jaw: a derived morphology in anuran tadpoles and its possible developmental origin. BioEssays 27:526–532
Thienemann A (1934) Der tierweot der tropischen Planzengewasser. Arch Hydrobiol Suppl 13:1–9
Trueb L, Diaz R, Blackburn DC (2011) Osteology and chondrocranial morphology of Gastrophryne carolinensis (Anura: Microhylidae), with a review of the osteological diversity of New World microhylids. Phyllomedusa 10:99–135
Vacher JP, Kok PJR, Rodrigues MT, Lima JD, Lorenzini A, Martinez Q, Fallet M, Curtois EA, Blanc M, Gaucher P, Dewyntier M, Jairam R, Ouboter P, Thébaud C, Fouquet A (2017) Cryptic diversity in Amazonian frogs: Integrative taxonomy of the genus Anomaloglossus (Amphibia: Anura: Aromobatidae) reveals a unique case of diversification within the Guiana Shield. Mol Phyl Evol 112:158–173
Van Bocxlaer I, Loader SP, Roelants K, Biju SD, Menegon M, Bossuyt F (2010) Gradual adaptation toward a range-expansion phenotype initiated the global radiation of toads. Science 327:679–682. https://doi.org/10.1126/science.1181707
Vera Candioti MF (2005) Morphology and feeding in tadpoles of Ceratophrys cranwelli (Anura: Leptodactylidae). Acta Zool 86:1–11
Vera Candioti MF, Úbeda C, Lavilla EO (2005) Morphology and metamorphosis of Eupsophus calcaratus tadpoles (Anura: Leptodactylidae). J Morphol 262:161–177
Vera Candioti MF, Brusquetti F, Netto F (2007) Morphological characterization of Leptodactylus elenae tadpoles (Anura: Leptodactylidae: L. fuscus group), from central Paraguay. Zootaxa 1435:1–17
Vera Candioti MF, Nuñez JJ, Úbeda C (2011a) Development of the nidicolous tadpoles of Eupsophus emiliopugini (Anura: Cycloramphidae) until metamorphosis, with comments on systematic relationships of the species and its endotrophic developmental mode. Acta Zool 92:27–45
Vera Candioti MF, Nuñez JJ, Ubeda C (2011b) Development of the nidicolous tadpoles of Eupsophus emiliopugini (Anura: Cycloramphidae) until metamorphosis, with comments on systematic relationships of the species and its endotrophic developmental mode. Acta Zool 92:27–45
Vera Candioti F, Grosso J, Haad B, Pereyra MO, Bornschein MR, Borteiro C, Costa P, Kolenc F, Pie MR, Proaño B, Ron S, Stanescu F, Baldo D (2016) Structural and heterochronic variations during the early ontogeny in toads (Anura: Bufonidae). Herpetol Monogr 30:79–118
Vera Candioti F, Grosso J, Pereyra MO, Haad MB, Lescano J, Siu-Ting K, Aguilar C, Baldo D (2020) Larval anatomy of Andean toads of the Rhinella spinulosa group (Anura: Bufonidae). Herpetol Monogr 34:116–130. https://doi.org/10.1655/HERPMONOGRAPHS-D-20-00001_hmon-34-01-05_11
Vera Candioti F, Dias PH, Rowley JJ, Hertwig S, Haas A, Altig R (2021) Anatomical features of the phytotelma dwelling, egg-eating, fanged tadpoles of Rhacophorus vampyrus (Anura: Rhacophoridae). J Morphol 282:769–778
Vera Candioti MF (2007) Anatomy of anuran tadpoles from lentic water bodies: systematic relevance and correlation with feeding habits. Zootaxa 1600, 1–175. https://doi.org/10.11646/zootaxa.1600.1.1
Viertel B (1982) The oral cavities of central European anuran larvae (Ampphibia) morphology, ontogenesis and generic diagnosis. Amphibia-Reptilia 4:327–360
Viertel B, Channing A (2017) The larva of Schismaderma carens (Smith, 1849) (Anura: Bufonidae) - a redescription. Alytes 33:38–46
Wake MH (2015) Fetal adaptations for viviparity in amphibians. J Morphol 276:941–960. https://doi.org/10.1002/jmor.20271
Wasserssug RJ (1976) Oral morphology of anuran larvae: terminology and general description. Occ Pap Mus Nat Hist Univ Kansas 48:1–23
Wassersug RJ (1972) The mechanism of ultraplanktonic entrapment in anuran larvae. J Morphol 137:279–287
Wassersug RJ (1980) Internal oral features of larval from eight anuran families: functional, systematic, evolutionary and ecological considerations. Misc Publ Univ Kansas Mus Nat Hist 68:1–146
Wassersug RJ, Duellman WE (1984) Oral structures and their development in egg-brooding hylid frog embryos and larvae: evolutionary and ecological implications. J Morphol 182:1–37
Wassersug RJ, Heyer WR (1988) A survey of internal oral features of leptodactyloid larvae (Amphibia: Anura). Smithsonian Contr Zool 457:1–99
Wassersug RJ, Rosenberg K (1979) Surface anatomy of branchial food traps of tadpoles: a comparative study. J Morphol 159:393–425
Yeh J (2002) The evolution of development: two portraits of skull ossification in pipoid frogs. Evolution 56:2484–2498
Acknowledgements
The authors would like to thank the Museu de História Natural of the Universidade Federal de Alagoas for allowing us to access the material; to Tamí Mott for all the support and encouragement of research with herpetofauna in the state of Alagoas. We are thankful to Martín Pereyra and to an anonymous reviewer for their comments and suggestions in an earlier version of the manuscript; any errors are our own. Marcos J. M. Dubeux thanks Fundação de Amparo a Ciência e Tecnologia do Estado de Pernambuco—FACEPE (IBPG-1117-2.04/19) for financial support. Specimens were collected under SISBio/ICMBIO collection permits n° 33507-1 and 74417-2). Pedro H. Dias would like to thank the Marie Sklodowska-Curie Actions (MSCA-IF-2020, MEGAN; 101030742). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Marcos JM Dubeux, Conceptualization, Formal analysis, Funding acquisition, Methodology, Visualization, Writing – original draft. Filipe AC Nascimento, Conceptualization, Formal analysis, Methodology, Visualization, Writing – original draft. Pedro HS Dias, Conceptualization, Formal analysis, Funding acquisition, Methodology, Visualization, Writing – original draft, Project administration.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare.
Ethics approval
No approval of research ethics committees was required to accomplish the goals of this study, because no experimental work was conducted. Tadpoles were collected under the permanent permit # 74417-2 issued to Tamí Mott by Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis (IBAMA) and Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) and deposited in a scientific collection.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary file1 (MP4 47589 KB)
Appendices
Appendix 1 Additional tadpoles studied
Taxa | External morphology | Buccopharyngeal cavity | Musculo-skeletal system |
|---|---|---|---|
Adenomus kelaartii | Haas et al. (1997) | ||
Amazophrynella minuta | This study | This study | This study |
Anaxyrus americanus | This study | – | This study |
Ansonia hanitschi | This study | This study | This study |
Atelopus nanay | This study | This study | – |
Barbarophryne brongersmai | This study | This study | – |
Bufo bufo | This study | Viertel (1982) | – |
Capensibufo tradouwi | Channing et al. (2012) | – | – |
Dendrophryniscus brevipollicatus | This study | This study | This study |
Duttaphrynus melanostictus | This study | This study | This study |
Frostius pernambucensis | This study | This study | This study |
Incilius coniferus | This study | This study | This study |
Ingerophrynus divergens | Inger (1985) | Inger (1985) | – |
Melanophryniscus klappenbachi | Baldo et al. (2014) | Baldo et al. (2014) | Baldo et al. (2014) |
Mertensophryne taitana | Müller et al (2005) | – | – |
Nannophryne variegata | This study | – | – |
Osornophryne occidentalis | Romero-Carvajal et al. (2023) | Romero-Carvajal et al. (2023) | – |
Peltophryne peltocephala | This study | This study | This study |
Poyntonophrynus fenoulheti | Lambris (1994) | ||
Rentapia hosii | Inger (1985) | Inger (1985) | |
Rhaebo glaberrimus | This study | – | This study |
Rhinella marina | This study | This study | This study |
Schismaderma carens | Viertel and Channing (2017) | – | |
Sclerophrys maculata | This study | This study | – |
Vandijkophrynus gariepensis | Channing et al. (2012) | Lambris (1994) | – |
Werneria mertensiana | This study | – | – |
Outgroup | |||
Macrogenioglottus alipioi | This study | This study | This study |
Odontophrynus cultripes | This study | This study | This study |
Proceratophrys appendiculata | This study | This study | This study |
Amazophrynella minuta (Melin, 1941): ICN 54915.
Anaxyrus americanus (Holbrook, 1836): AMNH 153168.
Atelopus nanay Coloma, 2002: QCAZ 3672.
Barbarophryne brongersmai (Hoogmoed, 1972): ZMH 12162.
Dendrophryniscus brevipollicatus Jiménez de la Espada, 1870: UNIRIO 3394.
Duttaphrynus melanostictus (Schneider, 1799): ZMH 13591.
Incilius coniferus (Cope, 1862): UCR 18999.
Macrogenioglottus alipioi MHNUFAL 10811.
Melanophryniscus spectabilis Caramaschi & Cruz, 2002: CFBH 3719.
Nannophryne variegata Günther, 1870: AMNH 81404.
Odontophrynus cultripes UFMG937.
Peltophryne peltocephala (Tschudi, 1838): AMNH 38451.
Proceratophrys appendiculata UNIRIO4036.
Rhaebo glaberrimus (Günther, 1869): ICN 49629.
Rhinella marina (Linnaeus, 1758): ICN 53853.
Sclerophrys maculata (Hallowell, 1854): ZMH 11955.
Werneria mertensiana Amiet, 1976: ZMB 79695.
Appendix 2: Character evolution
For the analysis of phenotypic evolution, we sample taxa based on Jetz and Pyron (2018) phylogenetic hypothesis. Characters were scored based on personal observation and/or literature information. We assumed the monophyly of Frostius and the sister relationship between F. erythrophthalmus and F. pernambucensis (see Materials and methods). We recognize that in the absence of data for the larvae of F. erythrophthalmus, our synapomorphies propositions are only putative, pending further investigation.
Phenotypic characters
Character 1: Number of tooth rows on the upper lip: one (0); two (1)
The typical tadpole has flexible, dermal ridges on both upper and lower lip; on top of those ridges, there are keratinous teeth, or keratodonts (Altig and McDiarmid 1999). The number and organization of these tooth row can vary drastically, reaching up to 17 superior and 21 inferior tooth rows (Altig and Johnston 1989). In bufonids, however, most species present the same number of rows in the upper (2) and in the lower (3) lips. Given that the number of rows in each lip seem to evolve independently (Altig 2006), we opted for coding each lip separately.
Taxonomic distribution and optimization: The examined larvae of Frostius pernambucensis possess a unique condition, with a single row in each lip. Variation of the 2/3 is also observed in other bufonids, such as 0/0 in Blythophryne beryet (Chandramouli et al. 2016) and 1/0 in Pelophryne spp (Inger 1960; Malkmus et al. 2002; Leong and Teo 2009); these taxa, however, were not present in the phylogenetic hypothesis used by us. Our optimization suggests that the presence of a single row in each lip is a synapomorphy of Frostius (Fgi. 8A).
Character 2: Number of tooth rows on the lower lip: one (0); three (1)
See Character 1
Taxonomic distribution and optimization: Our optimization suggests that the presence of a single row in each lip is a synapomorphy of Frostius (Fig. 8B).
Character 3: Lip morphology: “normal” (0); dermal fold (1); expanded (2)
The “normal” lips of tadpoles consist of a dermal structure bearing marginal papillae and the tooth ridges, usually larger than the upper labia, free on its edges. Although both marginal (and submarginal) papillae, as well as the ridges can vary significantly, the lips per se are more or less uniform in several taxa. Interesting variation have been reported, as the reduction of lips in microhylids (e.g., Haas 2003) and in endotrophic larvae (e.g., Randrianiania et al. 2011), or the enlarged, funnel-like structure of umbelliform tadpoles (e.g., Dias et al. 2019, 2021). In the examined bufonids, we observed besides the “normal” configuration, an enlarged lip in suctorial and/gastromyzophorous forms, and a tick dermal fold, as in Frostius.
Taxonomic distribution and optimization: while and enlarged lip evolved independently at least three times in bufonids—Atelopus, Ansonia, and Weneria—the modification into a dermal fold occurred just in Frostius, and therefore could be an additional synapomorphy for the genus (Fig. 8C).
Character 4: Number of infralabial papillae: one pair (0); two pairs (1)
The infralabial papillae are located in the buccal floor, right after buccal opening (Wassersug 1976); several functions have been suggested for those papillae (Wassersug 1980), but no conclusive evidence has been presented. Within bufonids and their closely related taxa, two main conditions have been reported in the literature: a single pair in bufonids (e.g., Vera Candioti et al. 2020), or two pairs in odontophrydis (e.g., Nascimento et al. 2013; Dias et al. 2014)—with some variation in specific taxa (e.g., Dias et al. 2019; Dias 2020).
Taxonomic distribution and optimization: Frostius pernambucensis, as well as all bufonids presented a single pair of infralabial papillae, a condition that optimized as a synapomorphy of Bufonidae (Fig. 8D).
Character 5: Number of lingual papillae: two (0); three (1); four (2)
Lingual papillae are located on the lingual bud of tadpoles (Wassersug 1976). Considerable variation has been reported in the number and arrangements of these papillae (e.g., Wassersug and Heyer 1988).
Taxonomic distribution and optimization: the optimization of this character proved ambiguous; Frostius pernambucensis, as well as Melanophryniscus klappenbachi presented three lingual papillae, contrasting with the most of other bufonids, which have four papillae. Additionally, the absence of these papillae in Osornophryne (Romero-Carvajal et al. 2023) and the lack of data for Oreophrynella, precluded the optimization of this characters (Fig. 9A). It is important to note that variation exists within Melanophryniscus (Baldo et al. 2014), so further studies are required. Also, Amazophrynella minuta had a unique condition among bufonids—two lingual papillae; this genus also should be investigated further.
Character 6: Papillae in the buccal roof: absent (0); present (1)
Both buccal roof and floor are, usually, covered with papillae of different size, shape, and morphology (Wassersug 1976, 1980). These papillae can be distributed in different regions, such as the pre-pocket area or delimiting the roof and floor arenas. In rare cases, such as in endotrophic (e.g., Wassersug and Duellman 1984) or macrophagous (e.g., Vera Candioti et al. 2021) larvae, these papillae are absent. Many bufonids lacked papillae in the buccal roof.
Taxonomic distribution and optimization: state 0 was present in several lineages, including Frostius, Atelopus, Osornophryne, Ansonia, and Amazophrynella. Besides that, this condition is unknown for Oreophrynella. This distribution of states rendered the optimization of this character ambiguous (Fig. 9B).
Character 7: Pustulations in the buccal roof: absent (0); present (1)
As for the buccal roof papillae, may taxa have pustulations in the buccal floor and roof; these pustulations can be scattered in the buccal floor, as in many taxa (e.g., Vera Candioti 2007), or form a dense field of pustulations, as in many stream dwellers (e.g., Dias et al. 2014; Montesinos et al. 2022).
Taxonomic distribution and optimization: the distribution and optimization of this character is quite similar to that of character 6, with exception of Amazophrynella, that presented pustulations (Fig. 9C).
Character 8: Pustulations in the buccal floor: absent (0); present (1)
See characters 7 for description and distribution (Fig. 9D).
Characters 9: Postnarial papillae: absent (0); present (1)
Postnarial papillae are located immediately posteriorly to narial opening and can vary in number, arrangements, and morphology (e.g., Wassersug and Heyer 1988; Dias et al. 2021). Very rarely, it is absent from tadpoles.
Taxonomic distribution and optimization: within bufonids, postnarial papillae were absent in Frostius, Osornophryne, and Amazophrynella. The optimization of this character suggests that the lack of these papillae could be a synapomorphy of Frostius (Fig. 10A). Also, if its absence is confirmed in Oreophrynella, it could be a synapomorphy for Osornophryne + Oreophrynella. Finally, it deserves further inspection in other Amazophrynella species.
Character 10: Median ridge: absent (0); present (1)
The median ridge stands at the end of the postnarial arena (Wassersug 1976, 1980); this feature usually has a triangular shape, but variation has been reported regarding its morphology (e.g., Dias et al. 2019). A putative function of the median ridge is to divide the water flow into left and right due to its medial location (Wassersug 1980). This structure has been reported absent in endotrophic larvae (e.g., Wassersug and Duellman 1984).
Taxonomic distribution and optimization: in our dataset, only two species lacked a median ridge within bufonids: Frostius pernambucensis and Osornophryne occidentalis and the optimization suggests that such absence could be a synapomorphy for Frostius (Fig. 10B).
Character 11 Lateral ridge papillae: absent (0); present (1)
The lateral ridge papillae are present in “type IV” tadpoles and can vary in size, shape, and morphology (e.g., Chou and Lin 1997). They are located laterally on the buccal roof, facing the buccal roof (Wassersug 1976).
Taxonomic distribution and optimization: similar to character 10 (Fig. 10C), but lateral ridge papillae are also absent in Ansonia.
Character 12: Secretory ridges: absent (0); present (1)
Secretory ridges are responsible for the production of mucus and pivotal for the functioning of the food traps of buccopharyngeal cavity (Kenny 1969; Wassersug 1972; Wassersug and Rosenberg 1979). They are located on the ventral surface of ventral velum and are characterized by an irregular, concentric pattern of ridges. Secretory ridges morphology usually varies accordingly to taxonomy and putative diet (Wassersug and Rosenberg 1979) and seem to be reduced or absent in macrophagous and endotrophic larvae.
Taxonomic distribution and optimization: within bufonids, the secretory ridges are absent in Frostius pernambucensis and Osornophryne occidentalis. Whereas the lack of data for Oreophrynella impacts the optimization regarding Osornophryne, the current optimization suggests that it could represent an additional synapomorphy of Frostius (Fig. 10D).
Character 13: Filter plates: absent (0); present (1)
The filter plates are the substrate for the gill filters, another essential structure involved in the food entrapment system of anurans (Kenny 1969; Wassersug 1972). A few studies dealt with these features in detail, but some evolutionary patters were identified; Wassersug (1980), for instance, suggested that there is a correlation between the number of filter rows and the capacity of filtering of a given tadpole. Macrophagous and/or endotrophic larvae have been reported with reduced filter plates (e.g., Wassersug and Duellmam 1984).
Taxonomic distribution and optimization: optimization of this character is similar to that of the secretory ridges and the absence of filter plates is a putative synapomorphy of Frostius (Fig. 11A).
Character 14: Muscle subarcualis rectus II–IV, origin: ceratobranchial IV (0); ceratobranchial III (1)
This small muscle usually runs from the ceratobranchil IV to the processus branchialis II of the ceratobranchial II (Haas 1997, 2003). Notwithstanding, it can be split in different portions, as in some hylids (e.g., Haas 2003; Dias et al. 2023), or having some fibers invading the interbranchial septum IV (e.g., Haas 2003; Vera Candioti et al. 2011a, b). In Frostius, a unique condition is present, and the muscle originates at the ceratobranchial III.
Taxonomic distribution and optimization: state 1 is present solely in Frostius (autapomorphic condition), and could be a synapomorphy for the genus, pending confirmation in F. erythrophthalmus (Fig. 11B).
Character 15: Muscle subarcualis rectus II–IV, insertion: ceratobranchial I (0); ceratobranchial II (1)
See character 14.
Taxonomic distribution and optimization: Frostius pernambucensis also have a unique condition for the insertion of the m. subarcualis rectus II–IV (Fig. 11C).
Character 16: Muscle subarcualis rectus I ventral a` slip, insertion: ceratobranchial II (0); ceratobranchial III (1)
Anurans may present up to three slips of the m. subarcualis rectus I; generally the dorsal slip inserts on the ceratobranchial I, and the two ventral slips (a and `a) on the ceratobranchials II and III, respectively (Haas 1997, 2003). Deviations from this pattern have been reported in many lineages (Haas 2003). We observed in Frostius pernambucensis that both ventral slips of the m. subarcualis rectus I are inserted in the ceratobranchial II.
Taxonomic distribution and optimization: state 0 is restricted to Frostius and it is a putative synapomorphy of the genus (Fig. 11D).
Character 17: Muscle levator arcuum branchialium IV, insertion: ceratobranchial IV (0); ceratobranchial III (1)
Anurans may present up to three levator arcuum branchialium. The levators I–IV usually inserts on the respective ceratobranchials (Haas 2003). Usually, there is some variation in the insertion pattern of the fourth levator, which can reach a distal or proximal portion of the ceratobranchial IV. In Frostius, there is a unique condition in which the levator IV inserts on the ceratobranchial III.
Taxonomic distribution and optimization: state 1 is restricted to Frostius and it is a putative synapomorphy of the genus (Fig. 12A).
Character 18: Muscle tympanopharyngeus, insertion: ceratobranchial IV (0); ceratobranchial III (1)
The tympanopharyngeus is closely associated with the levator arcuum branchialium IV, indistinguishable for it in some case (Haas 2003). In Frostius, there is a unique condition in which the tympanopharyngeus inserts on the ceratobranchial III.
Taxonomic distribution and optimization: state 1 is restricted to Frostius and it is a putative synapomorphy of the genus (Fig. 12B).
Character 19: Suprarostral alae and corpus: partially or completely separated (0); fused (1)
The suprarostral cartilages provide support for the upper jaw sheath. Usually, these cartilages have a tri- or tetrapartite condition, with the alae completely free or partially free from the corpora, which are very often continuously distally (Haas 1995, 2003). Different variation has been reported in that configuration (e.g., Dias et al. 2021), but a completely fused corpora is not common (e.g., Vera Candioti 2005; Dias et al. 2019). Frostius pernambucensis, as well as the suctorial larvae of Ansonia present a single-element corpora.
Taxonomic distribution and optimization: optimization of this character suggests that state 1 is a putative synapomorphy for Frostius (Fig. 12C).
Character 20: Suprarostral cartilage and cornua trabeculae: articulating (0); fused (1)
Suprarostral cartilages and the cornua trabeculae are articulating elements in the anuran larvae (Haas 1995, 2003). In Frostius, there is a stripe of cartilage fusing these two elements synchodroticaly. This condition has never been reported or observed in any other bufonid as far as we know.
Taxonomic distribution and optimization: optimization of this character suggests that state 1 is a putative synapomorphy for Frostius (Fig. 12D).
Character 21: Commissura quadratoorbitalis: absent (ligamentous) (0); present (1)
The commissura quadratoorbitalis is a cartilaginous connection between the processus muscularis and the processus anthorbitalis (Haas 1995). In several taxa, this cartilaginous bridge is not formed, and such a connection is made of ligamentous (Haas 2003). In most bufonids, the commissura is present (Haas 2003), although it has been previously reported absent, as in Melanophryniscus (Baldo et al. 2014). The commissura, however, is also present in bufonid`s sister taxa, Odontophrynidae (e.g., Nascimento et al. 2013; Dias 2020).
Taxonomic distribution and optimization: optimization of this character is ambiguous (Fig. 13A). It seems that it was lost early in the evolution of bufonids and regained in less inclusive clades. The current available evidence does not allow a test for this hypothesis.
Character 22: Ceratobranchial II and planum hypobranchiale: articulating (0); fused (1)
Anurans usually have four ceratobranchials, of which the first is fused to the planum hypobranchiale, whereas the others are articulating with it (Haas 1995, 2003). In Frostius, the ceratobranchials II and III (see character 23) are fused to the planum hypobranchiale, a unique condition among bufonids.
Taxonomic distribution and optimization: optimization of this character suggests that state 1 is a putative synapomorphy for Frostius (Fig. 13B).
Character 23: Ceratobranchial III and planum hypobranchiale: articulating (0); fused (1)
See character 22.
Taxonomic distribution and optimization: optimization of this character suggests that state 1 is a putative synapomorphy for Frostius (Fig. 13C).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dubeux, M.J.M., do Nascimento, F.A.C. & Dias, P.H.d. Larval morphology of Frostius pernambucensis (Anura): contribution of larval characters for the systematics of the family Bufonidae and evolution of endotrophic tadpoles. Zoomorphology 143, 159–187 (2024). https://doi.org/10.1007/s00435-023-00623-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-023-00623-6