Abstract
Existing techniques for examining the everted vesica (endophallus) of Lepidoptera are based primarily on cuticular preparations macerated with a caustic solution for taxonomic study. These techniques destroy muscles and other soft tissue, thus studies of the functional anatomy of the skeletomuscular apparatus of the phallus are not possible. Injection of formaldehyde solution into the phallus of fresh specimens is proposed as a new approach for studying the intact anatomy of this structure. The new technique results in simultaneously everting and fixing the vesica, resulting in a better approximation of its functional shape. This method produces properly fixed tissues and the whole structure can be processed for various further studies, including histology sectioning, scanning electron microscopy, and confocal laser scanning microscopy. The commonly used stubs for scanning electron microscopes do not allow observation of the sample from all aspects. This problem was solved by the modification of a commercially available stub. The device allows 360° rotation of the phallus, and the concept can be applied for observation of other objects as well.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The importance of studying male and female genitalia in Lepidoptera cannot be overstated. No other group of insects relies so much on the morphology of the genitalia for making taxonomic decisions. The integumentary parts of the male and female genitalia are easily accessible by conventional techniques generally based on alkaline digestion of soft organic matter and subsequent cleaning of the structures. The genitalia are then positioned between a microscope slide and a coverslip using a permanent mounting medium (e.g., Robinson 1976; Busck 1942 among many others). Depending on the skill of the technician and the morphological complexity of the taxon, this basic protocol, of which there are multiple variants, allows the study of practically any genital structure with a comparable standard, and the same techniques can be applied to museum specimens even if they are extremely old. Genitalia preparation techniques have also been modified to allow for DNA extraction during the same process, thus allowing for molecular identification of voucher specimens without the need to remove other tissue (Knölke et al. 2005). Although originally the phallus was studied externally, complementary technical solutions collectively known as vesica eversion techniques (Mikkola 2007) have been developed to improve access to its important internal structures (the endophallus or vesica). In some groups of large moths (e.g., Noctuidae), vesica eversion has become standard (Fibiger 1997), and in some microlepidoptera the vesica eversion has been applied with equal success (Dang 1993). However, these conventional approaches impose some serious limitations. First, slide mounting of an everted vesica introduces some artefacts because the structure must be compressed in some way between the microscope slide and the coverslip. This is especially important with regard to the phallus, as the vesica includes a series of diverticula spatially distributed (Zlatkov 2016) which requires several preparations from different angles which may not be easy to observe (Zlatkov 2018). Furthermore, these methods are restricted to skeletal structures, as the preparation does not retain the musculature layout. It has been shown that the muscles associated with the male genitalia have evolutionary implications, and their study is essential to understand the functional morphology of the genitalia (Kuznetsov and Stekolnikov 1998, 2001; Kristensen 1984, 2003; Nielsen and Kristensen 1989; Simonsen 2006). To visualize the muscle arrangements, some techniques have been described to dissect, fix and stain fresh material (De Benedictis and Powell 1989). However, apart from some abovementioned studies, the muscles associated with the endophallus have received almost no attention, presumably due to the technical limitation of combining a fixation-staining method with an eversion technique in fresh material. In this study, we present some methods that allow for the preparation and examination of both the skeletal and muscle structure of the phallus in some Lepidoptera. The methods require only a few specimens, reducing the need for an extensive series of specimens, and the preparations can be examined in detail under both scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM).
Materials and methods
Material examined
Male moths from various groups were gathered from the vicinity of Sofia city, Bulgaria by attraction to artificial light (160 W HWL bulb, Osram, powered by a portable electric generator). The following species were used in the experiments: Aethes francillana (Fabricius, 1794) (2 specimens), Agapeta hamana (Linnaeus, 1758) (2 specimens) (Tortricidae), Nomophila noctuella ([Denis & Schiffermüller], 1775) (1 specimen) (Crambidae), Agrius convolvuli (Linnaeus, 1758) (1 specimen) (Sphingidae), Heliomata glarearia ([Denis & Schiffermüller], 1775) (2 specimens) (Geometridae), Phragmatobia fuliginosa (Linnaeus, 1758) (Erebidae) (2 specimens), Acontia trabealis (Scopoli, 1763) (3 specimens), Tyta luctuosa ([Denis & Schiffermüller], 1775) (1 specimen), Heliothis viriplaca (Hufnagel, 1766) (4 specimens), Helicoverpa armigera (Hübner, [1808]) (6 specimens), Cirrhia icteritia (Hufnagel, 1766) (2 specimens), Mythimna albipuncta ([Denis & Schiffermüller], 1775) (1 specimen), Peridroma saucia (Hübner, [1808]) (1 specimen) (Noctuidae). The living moths were transported to the laboratory in transparent plastic 30 ml containers. The specimens were euthanized by placing a small cotton ball soaked with 3–5 drops of ethyl acetate in the container. After 5–20 min, the container was opened under a fume hood and the moth was set under a stereomicroscope.
Dissection
The abdomen of the moth was detached from the thorax using forceps. The rest of the body was squashed between cotton pads to assure it was dead and discarded. The abdomen was dissected under a stereomicroscope in a small Petri dish (ø = 50 mm) by tearing one of the pleurae along its entire length with two pointed forceps. Then, the genitalia were dissected by separating one of the valvae from its articulation or by removing the tegumen. The phallus was extirpated together with the bulbus ejaculatorius and most of the ductus ejaculatorius simplex (Online Resource 1). Dry museum specimens (Cyrrhia icteritia, 2 males; Heliothis viriplaca, 1 male) from the National Museum of Natural History, Sofia, were used to obtain traditionally prepared (i.e., macerated) phalli generally following Hardwick (1950).
Eversion
The phallus along with the ductus ejaculatorius was transferred to a clean Petri dish. Some of the phalli were submerged in a few drops of Ringer solution (Woodring and Blakeney 1980; Online Resource 2) to avoid desiccation; the others were manipulated in a dry dish. Two solutions were tested as injection substances: Ringer saline and 4% paraformaldehyde in phosphate buffer (PFA) (e.g., Barbosa et al. 2015). Eversion was performed by injection of either solution into the cavity of the phallus through a glass capillary (Fig. 1). In some cases, the vesica was everted in two steps: first with Ringer solution and then with a fixative (PFA). In other specimens, the phallus was everted directly with a fixative. The capillary was inserted into various structures to test the most appropriate location: ductus ejaculatorius simplex; bulbus ejaculatorius; junction of the ductus ejaculatorius at the dorsal or posterior side (depending on the structure of phallus); coecum; and distal part of the sclerotised phallic tube. After insertion of the capillary, a gentle pressure was applied to the plunger of the syringe until the entire vesica was everted. The pressure was maintained for approximately one minute to block the reverse contracting ability of the muscle fibers, otherwise the muscles would retract the vesica. If the phallus was ejected from the capillary, it was re-inserted in the initial perforation and injected again. After eversion, the pressure was maintained for 15–40 min (depending on the size) until the tissues were partially fixed and any retractions and contractions ceased. This was accomplished in two different manners: 1) the syringe with the capillary inserted into the phallus was attached to a laboratory stand with a clamp in a vertical position and the phallus was submerged into a tube with fixative; 2) the syringe was in horizontal position and a dish was set below the capillary to collect the leaking liquid; the small leakage of fixative around the capillary kept the phallus wet. In both cases, pressure on the plunger was applied periodically (e.g., every minute). Because the syringe was filled with air (only the capillary was filled with liquid), there was always some remaining pressure which was transferred to the liquid into the phallus.
Sometimes considerable leakage of solution from the proximal end of the severed ductus ejaculatorius simplex occured (e.g., H. viriplaca). In these cases, a ligature on the bulbus ejaculatorius was applied. The ligature was prepared from human scalp hair (longer than 10 cm to ease the manipulation).
Fixation and storage of samples
After initial fixation by PFA injection, the phallus was transferred in a fresh fixative for 40–60 min. Further dissection was performed in a staining block with fixative under a stereomicroscope set in a fume hood. The ductus ejaculatorius was severed near the sclerotised phallic tube with micro scissors, and for the large phalli a small piece of the ventral side of phallus was removed with a micro knife to achieve better penetration of liquids. On larger vesicae, 2–3 small cuts were made for the same purpose. The phallus was placed in fixative at 4 °C for 4–7 days, then washed three times for 6–24 h with 0.1 M phosphate buffer with 0.3% Triton X-100. After washing, the samples were transferred for 3 h in 30% and 50% ethanol on a rotator, and eventually stored in 70% ethanol.
Preparation of capillary and syringe
For injection, self-made glass capillaries were used. They were prepared from laboratory glass pipettes (ø = 5 mm) cut to 60 mm pieces. One of the ends was glued with an epoxy glue (Poxipol®) to a Luer fitting from a disposable syringe needle. Before that, the narrow end of the fitting was cut with a knife. The free end of the glass tube was heated with a laboratory gas burner until the glass started to melt, then with forceps was pulled quickly. The procedure usually should be repeated several times until the desired capillary diameter was obtained. The final capillary diameter depended on the diameter of the phallus to be injected: 0.20 mm for larger phalli (e.g., H. viriplaca, A. convolvuli) and 0.05 mm for smaller phalli (e.g., A. francillana, H. glarearia). The size was adjusted under a stereomicroscope by breaking the capillary at the desired diameter. The capillary was filled with a solution by a syringe with a dispensing needle as long as its widest Section (70–80 mm). The glass capillary was then screwed to a 2 ml disposable syringe with Luer lock (KD-Ject®), with the plunger pulled out in advance. Some pressure was applied on the plunger to remove the remaining air in the capillary tip, then the Luer cone was unlocked to release the air pressure in the syringe and locked again. Immediately before the injection, the pressure in the syringe was reduced by pulling the plunger by ca. 0.5 divisions on the syringe barrel to avoid unwanted leakage from the capillary tip.
Scanning electron microscopy (SEM)
Samples destined for SEM received extra fixation by overnight immersion in Karnovsky’s fixative. Samples were thoroughly washed with water and progressively dehydrated in ethanol. Samples were placed inside microporous specimen capsules (30 μm pore size) immersed in absolute ethanol, followed by critical point drying in an Autosamdri 814. The phalli were stabbed through the coecum along the phallic tube with a specially prepared stub (see next paragraph), glued with a conductive silver paste to the needle of the holder, and coated with AuPd using a Sputter Coater Polaron SC7640 for 100 s (approximately at a rate of 3 Å/s). The needle was rotated 180º and the sample coated again. The observation was carried out in a Scanning Electron Microscope Hitachi S‐4800 at the electron microscopy service of the University of València (SCSIE).
Preparation of stubs for scanning electron microscope
An aluminium stub for a Hitachi scanning electron microscope (product No.16333, Ted Pella Inc.) was customized to hold the samples. A small radial hole (ø = 0.5 mm) was drilled near the top. Another hole was drilled in a way to cross the previous one; it accommodated a stopper screw (M2). Two types of modifications were tested: (1) with the small hole at 4 mm below the top and a screw on the top side of the stub, parallel to the axis of the cylinder; and (2) with the small hole at 2 mm below the top and a side screw, perpendicular to the axis of the cylinder; a part of the upper section of the stub was removed to free space for the stopper screw (Figs. 2, 3). An auxiliary sample holder was made from a 27G syringe needle and a 0.2 mm minuten needle inserted into the previous one. The syringe needle tip was slightly blunted with a fine abrasive and cut at 9 mm. The minuten was shortened to ca. 12 mm, bent slightly at two points to assure tightening and inserted into the cut end of the syringe needle in a way that only 3 mm from the tip remained outside. After the attachment of the sample to the minuten tip, the holder was inserted into the small hole of the modified aluminium stub and the stopper screw was tightened. The original tip of the syringe needle projected from the outer wall of the stub and was used as a key to rotate the sample holder axially. A special screwdriver was made from the same size of syringe needle (27G) inserted into a piece of a 22G syringe needle, both compressed together to tighten the contact between them. The Luer fitting of the 27G needle was glued with hot glue into a piece of 2 ml plastic tube serving as a handle. Positions indicating axial rotation were inscribed onto the seal of the tube.
Technical drawing of modified aluminium stub for SEM for observation of the phallus in Lepidoptera (left), holder for sample attachment made from minuten 0.2 mm and hypodermic needle 27G (upper right) and screwdriver for rotation of the holder made from hypodermic needles 22G, 27G and plastic 2 ml centrifuge tube (bottom right)
Sectioning and light microscopy
To test the quality of this fixation technique for histological purposes, the vesicae of three species (H. armigera, H. viriplaca and A. trabealis) were embedded in Spurr epoxy resin (Spurr 1969) following the method of Pernstich et al. (2003). Some samples were dehydrated with ethanol according to the original protocol (Spurr 1969), but the result was not satisfactory because of incomplete polymerisation. The larger vesicae (e.g., H. armigera) were cut into small pieces (ca. 3 × 3 mm), each embedded in a separate resin block. The resin blocks were sectioned at 1 μm with a paraffin microtome equipped with a glass knife and modified according to Semba (1979). The sections were affixed to microscope slides and stained following Pernstich et al. (2003). Selected slides were sealed with Euparal and photographed immediately through an Amplival (Carl Zeiss Jena) compound microscope. The cuticular preparations of everted vesicae were submerged in absolute ethanol, attached to a pin inserted through the “opening” of the sclerotised phallus, and photographed under a Stemi 2000-c (Carl Zeiss) stereomicroscope. Helicon Focus (HeliconSoft) was used for z-stacking of the images.
Confocal laser scanning microscopy (CLSM)
To visualize the muscles, the samples stored in ethanol were re-fixed in 4% paraformaldehyde in 0.2 M phosphate buffer with 0.3% Triton X-100 (PBST) at room temperature for one hour. The samples were washed several times in PBST, at least 2 h, and stained with Texas Red™-X Phalloidin (1:50, Thermo Fisher Scientific, #00033) following Siwanowicz and Burrows (2017) at 4 °C with agitation for 4–5 days. The samples were then washed several times with PBST for at least 4 h. Preparations were mounted semi-permanently in FluorSave™ Reagent (Sigma-Aldrich, #345789) to previsualize under a fluorescent microscope (Nikon Eclipse 800) or permanently in Euparal to visualize under confocal laser scanning microscopy (Olympus FV1000) at 10X and 20X. To maximize the final image resolution, and avoid auto-fluorescence of the cuticle, the samples were subjected to a dehydration process, starting with ethanol 10% to absolute ethanol. Finally, the samples were cleared overnight in methyl salicylate 98% and then transferred to Euparal. All images were digitally edited with Photoshop (Adobe) software. The line drawings were prepared with Illustrator (Adobe). The CLSM images were obtained and stacked with FluoView (FV10-ASW) software.
Results
The dissection of the abdomen is best accomplished in a small dish (e.g., Petri dish or staining block; Online Resource 1). Rapid manipulation is necessary to avoid drying and other changes to the tissues. An experienced researcher can dissect the abdomen and remove the phallus within a minute. The excised phallus, particularly if it is a small one, desiccates quickly and may be submerged into a Ringer solution (Woodring and Blakeney 1980). This recipe was used by Zwick (2009) for the dissection of the genitalia of fresh moth material. In most cases, this solution does not cause visible osmotic effects on the tissues and contractions of the musculature. However, in general, the use of the Ringer solution should be avoided when possible, because of the considerable variation of the osmolarity of the haemolymph of different species and unpredictable effects on the muscles.
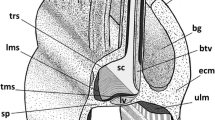
Various techniques for injection were tested prior to establishing the proposed one. The most important detail is the area of insertion of the capillary. In all cases the capillary must be inserted distally of the bulbus ejaculatorius (Fig. 1), otherwise eversion is not achieved. The dorsal connection of the ductus ejaculatorius appears a trouble-free area because it is devoid of muscle attachments (no important structures that can be damaged), and it is easily perforated with a glass capillary. The insertion of the capillary directly into the sclerotised phallic tube proved to cause problems: breaking the capillary, cracking the cuticle around the perforation and causing leakage of liquid, and damage to muscles. Only the external integument must be perforated with the capillary. In the case of perforation of the vesica proper, eversion is no longer possible. Eversion by injection of Ringer solution is easy, but reversible: the muscle retracts the vesica in only a few seconds after removing the capillary. The best eversion is achieved by direct injection of fixative. An important step is maintaining stronger pressure for enough time (e.g., 30 s) allowing the fixative to extend the folds and immobilize the internal phallic muscle, which is contractible a long time after the separation of the phallus from the body. The speed of injection is crucial for the success of the eversion: if not performed within the first few seconds, the endophallic musculature may contract and hamper complete eversion. Sometimes excessive pressure may be applied in an attempt to succeed in the eversion, but this often detaches the muscle fibers from their insertion points. Prolonged anaesthesia enhances muscle relaxation but should not be too long particularly for ultrastructural studies, because post-mortem changes may commence. To ensure good inflation of the vesica, some pressure should be maintained after the initial injection. For smaller specimens (e.g., Pyralidae, smaller Geometridae), 15 min is enough time, for larger (e.g., most Noctuidae), 30–40 min is necessary. If the fixative in the capillary lowers rapidly because of leakage, the syringe can be screwed off and the capillary reloaded as many times as necessary; this should be done without removing the phallus if possible.
The injection tool consists of a glass capillary with a Luer fitting and a syringe. The commercially available capillaries have small volumes; therefore, self-made ones appear to be more useful. Only the capillary should be filled with liquid, the syringe is filled with air. In this way, controlling the pressure of the system is easier. Some pressure remains in the barrel even after a short push on the plunger, and it is enough to maintain the vesica inflated for minutes.
The two modifications of the SEM stubs provided different results. The stub with a top screw and small hole at 4 mm below the top turned out to be ineffective because of the long distance between the sensors of the microscope and the sample; it did not allow focusing at high magnification. The second modification (illustrated in Figs. 2, 3) was more convenient and allowed for proper focusing. The noctuid C. icteritia was selected as a model to compare the everted vesica after maceration and after PFA injection. The macerated vesica appeared larger, completely everted, with all cornuti exposed (Fig. 4). In contrast, the PFA injected ones are smaller due to some non-everted folds, with diverticula and cornuti in different positions in comparison to the macerated vesicae. The position of the large cornutus is strikingly different: in the PFA fixed vesica it is sunk within the gonopore and only a half is exposed outside. Numerous muscle fibers are attached around its base. Both the PFA-injected specimens showed the same positions of cornuti and diverticula (Figs. 5, 6).
Distal part of the phallus of Cirrhia icteritia (Noctuidae) macerated with 10% KOH solution stained with chlorazol black and vesica inflated with absolute ethanol (light microscopy). The vesica of this species has three types of fixed cornuti attached to different areas. The black conical object is the tip of an insect pin. a Dorsal view. b Right view. c Ventral view. d Left view. 1 small cornutus; 2 large cornutus; 3 group of small cornuti (spinules). Scale bar 1 mm
Distal part of the phallus of Cirrhia icteritia (Noctuidae) fixed by injection of buffered PFA solution (SEM). a Dorsal view. b Right view. c Ventral view. d Left view. Note the difference in the general shape in comparison with the previous figure. For other explanations see the legend of Fig. 4. Scale bar 1 mm
Phallus of Cirrhia icteritia (Noctuidae) fixed by injection of buffered PFA solution and intrinsic phallic musculature: stained with Texas Red™-X Phalloidin (CLSM: a, b) and under SEM (c). a Whole phallus. b Detail of diverticulum with mesh of muscle fibres. Cornuti coloured digitally in green. c Muscle fibres exposed after partial removing of the integument, specimen from Fig. 5. For other explanations see the legend of Fig. 4. Scale bars a 1 mm, b, c 250 μm
Discussion
The proposed technique for simultaneous eversion and fixation produces properly fixed tissues (Fig. 7) and preserves the three-dimensional structure of the phallus. It can be assumed that phalli fixed in this way are closer to their real functional condition than cuticular preparations. The macerated phalli, after maximal eversion and inflation, sometimes appear with long diverticula bearing large spines on their tips, but this condition is most probably unnatural. For example, when macerated, the vesica of Eugnosta magnificana (Rebel, 1914), has two voluminous diverticula with long spines (cornuti) emerging from their tips, but it is found that in the intact phallus only a short part of the diverticula is everted and the basal 2/3 of the cornuti are retained within the vesica (Zlatkov 2018). A similar condition is observed in C. icteritia and only half of the longest cornutus is exposed outside in the PFA injected specimens. The different appearance of the macerated vesica is due to the fact that the intrinsic phallic muscles and the epidermis are completely removed during maceration, and thus nothing remains to hold the cuticular structures in their natural position. Another important advantage of this method for injection is the rigidity of the formaldehyde-fixed vesica. The shape of macerated vesica is preserved by rapid injection with a strong dehydrating agent (e.g., absolute ethanol), and is prone to deformation and desiccation during manipulation. These problems are solved after formaldehyde fixation and the vesicae are easily manipulated in various liquid media.
Sections of the vesica of Heliothis viriplaca fixed by injection of buffered formaldehyde solution. a General view of a section through the medial part of the vesica. b Detail of a, numerous muscle fibres, mostly cross-sectioned, and some tracheae are scattered in the vesica. c Attachment area of three fibres of the retractor muscle. d Thickened cuticle with acanthae. 1 muscle fibres; 2 tracheae; 3 epidermis; 4 endocuticle; 5 tendon cell; 6 exocuticle. Scale bars a 200 μm, b 100 μm, c and d 10 μm
The injection with fixative is not the only available technique providing intact everted phalli. Dang (1993) proposed the treatment of living moths with the insecticide 2,2-dichlorovinyl dimethyl phosphate, which in certain doses causes eversion of the vesica. This technique, though originally proposed for cuticular preparations, has been successfully applied for the examination of the functional anatomy of the phallus of E. magnificana. It has some constraints, particularly the difficult dosage, and the eversion is often interrupted at an initial stage because of poisoning of the moth (Zlatkov 2018). Regarding the low permeability of the cuticle to water solutions, fixation with PFA of everted phalli obtained in this way is presumably more difficult than with the injection technique.
The modified SEM stub and screwdriver for axial rotation of the samples allow for studying the phalli from various aspects (Fig. 5). Typically, standard stubs are used along with carbon adhesive tabs and the samples are attached permanently. In this way, the examination of the sample from all sides is not possible. The proposed modification of an aluminium stub overcomes this problem. It is adapted for a particular model of the electron microscope and sample size, but various shapes and sizes of stubs could be modified for the same purpose. The innovative modification is the small rotating axle for sample attachment. It can be used for other insect structures (e.g., bursa copulatrix) with the same success. No separation of the epidermis from the cuticle was observed (Fig. 7), i.e., the tissues appear properly fixed. The buffered 4% formaldehyde has long been applied to insect tissues and provides excellent results, but only when the tissues are directly exposed to the fixative because the cuticle has low permeability for this solution (Barbosa et al. 2015). The new injection technique allows studying the functional anatomy of the lepidopterous phalli by various methods, for example, histology sectioning, confocal microscopy (Fig. 6) and three-dimensional reconstructions of the internal phallic musculature. It is likely that other fixative formulas could be applied with the same success.
The success of injecting fixatives into the phallus depends mainly on the skills of the researcher. Fast manipulation is essential to avoid desiccation and other post-mortem changes to the tissues. Proper perforation of the integument with the capillary is a crucial step in the process. An important decision is the purpose of fixation: if a general anatomical study is the goal, prolonged treatment with anaesthetic is beneficial for better muscle relaxation; if the aim is a histological or ultrastructural examination, a short period of anaesthesia is more appropriate, though the vesica may remain incompletely everted.
References
Barbosa P, Berry DL, Kary CS (2015) Insect Histology: Practical Laboratory Techniques. Wiley, Hoboken, p 384
Busck A (1942) On the making of genitalia slides of Lepidoptera. Proc Hawaii Entomol Soc 11(2):157–163
Dang PT (1993) Vesicas of selected tortricid and small lepidopterous species, with descriptions of new techniques of vesica eversion (Lepidoptera: Tortricidae, Oecophoridae, Gelechiidae, and Nepticulidae. Can Entomol 125:785–789. https://doi.org/10.4039/Ent125785-5
De Benedictis JA, Powell JA (1989) A procedure for examining the Genitalic musculature of Lepidoptera. J Lepidopterist’s Soc 43(3):239–243
Fibiger M (1997) Noctuidae Europaeae volume 3 Noctuinae III. Entomological Press, Soro
Hardwick DF (1950) Preparation of slide mounts of Lepidopterous Genitalia. Can Entomol 82:231–235
Knölke S, Erlacher S, Hausmann A, Miller AM, Segerer AH (2005) A procedure for combined genitalia dissection and DNA extraction in Lepidoptera. Insect Syst Evol 35:401–409. https://doi.org/10.1163/187631204788912463
Kristensen NP (1984) The male genitalia of Agathiphaga (Lepidoptera: Agathiphagidae) and the lepidopteran groundplan. Entomol Scand 15:151–178. https://doi.org/10.1163/187631284X00127
Kristensen NP (2003) Skeleton and muscles: adults. In: Kristensen N (ed) Lepidoptera, moths and butterflies, vol 2. Morphology, physiology, and development. Walter de Gruyter, Berlin, pp 39–131. https://doi.org/10.1515/9783110893724.39
Kuznetzov VI, Stekolnikov AA (1998) Evolution of the skeleton and muscles of male genitalia in the families Riodinidae and Lycaenidae (Lepidoptera). Entomol Obozr 77:443–461 (in Russian)
Kuznetsov VI, Stekolnikov AA (2001) New approaches to the system of Lepidoptera of the World fauna (based on abdominal functional morphology). Nauka, St. Petersburg, Russia, p 462 (in Russian)
Mikkola K (2007) The rise of eversion techniques in lepidopteran taxonomy (Insecta: Lepidoptera). SHILAP Revta Lepid 35(139):335–345
Nielsen ES, Kristensen NP (1989) Primitive Ghost Moths. Monographs on Australian Lepididoptera. CSIRO Publishing, Clayton, pp 1–206
Pernstich A, Krenn HW, Pass G (2003) Preparation of serial sections of arthropods using 2, 2-dimethoxypropane dehydration and epoxy resin embedding under vacuum. Biotech Histochem 78(1):1–5. https://doi.org/10.1080/10520290312120002
Robinson GS (1976) The Preparation of Slides of Lepidoptera Genitalia with Special Reference to the Microlepidoptera. Entomol Gaz 27:127–132
Semba R (1979) Contributions to semithin sectioning on a conventional rotary microtome. Stain Technol 54(5):251–255. https://doi.org/10.3109/10520297909110680
Simonsen TJ (2006) The male genitalia segments in fritillary butterflies: comparative morphology with special reference to the ‘rectal plate’ in Issoria (Lepidoptera: Nymphalidae). Eur J Entomol 103:425–433
Siwanowicz I, Burrows M (2017) Three dimensional reconstruction of energy stores for jumping in planthoppers and froghoppers from confocal laser scanning microscopy. Elife. https://doi.org/10.7554/eLife.23824
Spurr A (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43. https://doi.org/10.1016/s0022-5320(69)90033-1
Woodring JP, Blakeney EW (1980) The role of free amino acids in osmoregulation of cricket blood (Acheta domesticus). J Insect Phys 26(9):613–618. https://doi.org/10.1016/0022-1910(80)90030-X
Zlatkov B (2016) On the vesica of Eucosmini and Grapholitini (Insecta: Lepidoptera: Tortricidae). Zootaxa 4168(2):297–312. https://doi.org/10.11646/zootaxa.4168.2.4
Zlatkov B (2018) Functional anatomy of the vesica in Eugnosta magnificana (Insecta: Lepidoptera: Tortricidae). Zoomorphology 137:535–544. https://doi.org/10.1007/s00435-018-0411-1
Zwick A (2009) The principal structure of male genital sclerites and muscles of bombycoid moths, with special reference to Anthelidae (Lepidoptera: Bombycoidea). Arthropod Struct Dev 38:147–161. https://doi.org/10.1016/j.asd.2008.07.006
Acknowledgements
We are most grateful to Todd Gilligan (Fort Collins, Colorado, USA) for his comments and corrections on an earlier draft of the manuscript. We also express our gratitude to Thomas Simonsen (Denmark) and an anonymous reviewer for constructive critical comments on the manuscript. This study was supported by the National Science Fund of Bulgaria, grant No. КП-06-H31/4–10.12.2019.
Funding
The study was supported by the National Science Fund of Bulgaria via grant No. КП-06-H31/4–10.12.2019.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation was performed by Boyan Zlatkov, Vladislav Vergilov, José Vicente Pérez Santa-Rita and Ognyan Sivilov. The first draft of the manuscript was written by Boyan Zlatkov and Joaquín Baixeras and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
No approval from research ethics committees was required to accomplish the goals of this study because experimental work was conducted with unregulated invertebrate species.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Dissection of abdomen and genitalia and injection of phallus of Heliothis viriplaca (WMV 93256 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zlatkov, B., Vergilov, V., Sivilov, O. et al. New approaches for studying the functional anatomy of the phallus in Lepidoptera. Zoomorphology 141, 335–345 (2022). https://doi.org/10.1007/s00435-022-00566-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-022-00566-4