Abstract
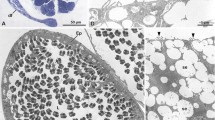
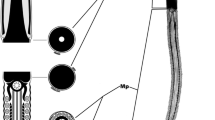
The spermatogenesis of the proturan Acerentomon microrhinus Berlese, (Redia 6:1–182, 1909) is described for the first time with the aim of comparing the ultrastructure of the flagellated sperm of members of this taxon with that of the supposedly related group, Collembola. The apical region of testes consists of a series of large cells with giant polymorphic nuclei and several centrosomes with 14 microtubule doublets, whose origin is likely a template of a conventional 9-doublet centriole. Beneath this region, there are spermatogonial cells, whose centrosome has two centrioles, both with 14 microtubule doublets; the daughter centriole of the pair has an axial cylinder. Slender parietal cells in the testes have centrioles with nine doublet microtubules. Spermatocytes produce short primary cilia with 14 microtubule doublets. Spermatids have a single basal body with 14 microtubule doublets. Anteriorly, a conical dense material is present, surrounded by a microtubular basket, which can be seen by using an α-anti-tubulin antibody. Behind this region, the basal body expresses a long axoneme of 14 microtubule doublets with only inner arms. An acrosome is lacking. The nucleus is twisted around the apical conical dense structure and the axoneme; this coiling seems to be due to the rotation of the axoneme on its longitudinal axis. The posterior part of the axoneme forms three turns within the spermatid cytoplasm. Few unchanged mitochondria are scattered in the cytoplasm. Sperm consist of encysted, globular cells that descend along the deferent duct lumen. Some of them are engulfed by the epithelial cells, which thus have a spermiophagic activity. Sperm placed in a proper medium extend their flagellar axonemes and start beating. Protura sperm structure is quite different from that of Collembola sperm; and on the basis of sperm characters, a close relationship between the two taxa is not supported.










Similar content being viewed by others
References
Abele LG, Kim W, Felgenhauer BE (1989) Molecular evidence for inclusion of the phylum Pentastomida in the Crustacea. Mol Biol Evol 6:685–691
Alberti G (1980) Zur feinstruktur der spermien und spermiocytogenese der Milben (Acari). II. Actinotrichida. Zool Jb Anat 104:144–203
Alberti G (2000) Chelicerata. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of Invertebrates. 9B Oxford and IBH Publishing Co Queensland, pp 311–388
Alberti G, Peretti AV (2002) Fine structure of male genital system and sperm in Solifugae does not support a sister-group relationships with Pseudoscorpiones (Arachnida). J Arach 30:268–274
Baccetti B, Dallai R, Fratello B (1973) The “12 + 0”, “14 + 0” or flagellate sperm of Protura. J Cell Sci 13:321–335
Beisson J, Wright M (2003) Basal body/centriole assembly and continuity. Curr Op Cell Biol 15:96–104
Berlese A (1909) Monografia dei Myrientomata. Redia 6:1–182
Bitsch C, Bitsch J (1998) Internal anatomy and phylogenetic relationships among apterygote insects clades (Hexapoda). Ann Soc Entomol France (NS) 34:339–363
Bitsch C, Bitsch J (2000) The phylogenetic interrelationships of the higher taxa of apterygote hexapods. Zool Scripta 29:131–156
Bitsch C, Bitsch J (2004) Phylogenetic relationships of basal hexapods among the mandibulate arthropods: a cladistic analysis based on comparative morphological characters. Zool Scr 33:511–550
Boore JL (1999) Animal mitochondrial genomes. Nucleic Acid Res 27:1767–1780
Boore JL, Collins TM, Stanton D, Daheler LL, Brown WM (1995) Deducing the pattern of arthropod phylogeny from mitochondrial DNA rearrangements. Nature 376:163–165
Callaini G, Dallai R (1993) The spermatozoon of Pseudoscorpions (Arachnida). Boll Accad Gioenia Sci Nat 26:35–52
Callaini G, Riparbelli MG, Dallai R (1999) Centrosome inheritance in insects: fertilization and parthenogenesis. Biol Cell 91:355–366
Carapelli A, Nardi F, Dallai R, Frati F (2006) A review of molecular data for the phylogeny of basal hexapods. Pedobiologia 20:191–204
Carapelli A, Liò P, Nardi F, van der Wath E, Frati F (2007) Phylogenetic analysis of mitochondrial protein coding genes confirms the reciprocal paraphyly of Hexapoda and Crustacea. BMC Evol Biol 7:S8
Carson HL (1945) A comparative study of the apical cell of the insect testis. J Morphol 77:141–161
Cook CE, Smith ML, Telford MJ, Bastianello A, Akam M (2001) Hox genes and the phylogeny of the arthropods. Curr Biol 11:759–763
Cook CE, Yue Q, Akam M (2005) Mitochondrial genomes suggest that hexapods and crustaceans are mutually paraphyletic. Proc R Soc B272:1295–1304
Dallai R (1970) The spermatozoon of Arthropoda. XI. Further observations on Collembola. In: Baccetti B (ed) Comparative spermatology. Academy Press, New York-London, pp 276–279
Dallai R (1991) Are Protura really Insects? In: Simonetta AM, Morris SC (eds) The early evolution of metazoa and the significance of problematic Taxa. Cambridge University Press, Cambridge, pp 263–269
Dallai R, Afzelius BA (1980) Characteristics of the sperm structure in Heteroptera (Hemiptera, Insecta). J Morphol 164:301–309
Dallai R, Afzelius BA (1999) Accessory microtubules in insect spermatozoa: structure, function and phylogenetic significance. In: Gagnon C (ed) The male gamete. From basic science to clinical applications. Coche River Press, Vienna, pp 333–350
Dallai R, Yin WY (1983) Sperm structure of Sinentomon (Protura) and phylogenetic considerations. Pedobiologia 25:313–316
Dallai R, Yin WY, Xué L, François J (1989) The gut structure of Sinentomon erythranum Yin (Protura: Sinentomidae). J Insect Morphol Embryol 18:173–184
Dallai R, Yin WY, Xué L (1990) Aflagellated spermatozoa of Huhentomon and Acerella (Protura, Apterygota). Int J Insect Morphol Embryol 19:211–217
Dallai R, Xué L, Yin WY (1992) Flagellate spermatozoa of Protura (Insecta, Apterygota) are motile. Int J Insect Morphol Embryol 21:137–148
Dallai R, Fanciulli PP, Frati F, Paccagnini E, Lupetti P (2003) Membrane specializations in the spermatozoa of collembolan insects. J Struct Biol 142:311–318
Dallai R, Fanciulli PP, Frati F, Paccagnini E, Lupetti P (2004) Sperm winding in Collembola. Pedobiologia 48:493–501
Dallai R, Lupetti P, Mencarelli C (2006) Unusual axonemes of hexapod spermatozoa. Int Rev Cytol 254:45–99
Dallai R, Zizzari ZV, Fanciulli PP (2008a) Fine structure of the spermatheca and of the accessory glands in Orchesella villosa (Collembola, Hexapoda). J Morphol 269:464–478
Dallai R, Zizzari ZV, Fanciulli PP (2008b) The ultrastructure of the spermathecae in the Collembola Symphypleona (Hexapoda). J Morphol 269:1122–1133
Dallai R, Zizzari ZV, Fanciulli PP (2009) Different sperm number in the spermatophores of Orchesella villosa (Geoffroy) (Entomobryidae) and Allacma fusca (L.) (Sminthuridae). Arthtropod Struct Develop 38:227–324
Davis EE, Brueckner M, Katsanis N (2006) The emerging complexity of the vertebrate cilium: new functional roles for an ancient organelle. Devel Biol 11:9–19
Dell’Ampio E, Szucsich N, Carapelli A, Frati F, Steiner G, Steinacher A, Pass G (2009) Testing for misleading effects in the phylogenetic reconstruction of ancient lineages of hexapods: influence of character dependence and character choice in analyses of 28S rRNA sequences. Zool Scr 38:155–170
Dutcher SK (2007) Finding treasures in frozen cells: new centriole intermediates. Bioessays 29:630–644
Giribet G, Richter S, Edgecombe, GD, Wheeler WC (2005) The position of crustaceans within the Arthropoda—evidence from nine molecular loci and morphology. In: Koenemann S, Jenner RA (eds) Crustacean Issues 16: Crustacea and Arthropod relationships. Festschrift for Frederick R. Schram. Taylor and Francis, Boca Raton, pp 307–352
Friederich M, Tautz D (1995) Ribosomal DNA phylogeny of the major extant arthropod classes and the evolution of myriapods. Nature 376:165–167
Fukui M, Machida R (2006) Embryonic development of Baculentulus densus (Imadaté): its outline (Hexapoda: Protura, Acerentomidae). Proc Arthropodan Embryol Soc Jpn 41:21–28
Fuller MT (1998) Genetic control of cell proliferation and differentiation in Drosophila spermatogenesis. Cell Dev Biol 9:433–444
Giribet G, Ribera C (2000) A review of arthropod phylogeny: new data based on ribosomal DNA sequences and direct character optimisation. Cladistics 16:204–231
Giribet G, Edgecombe GD, Carpenter GM, D’Haese CA, Wheeler WC (2004) Is Ellipura monophyletic? A combined analysis of basal hexapod relationships with emphasis in the origin of insects. Organ Divers Evol 4:319–340
González C, Tavosanis G, Molinari C (1998) Centrosomes and microtubule organization during Drosophila development. J Cell Sci 111:2697–2706
Hardy RW, Tokuyasu KT, Lindsley DL, Garavito M (1979) The germinal proliferation center in the testis of Drosophila melanogaster. J Ultrastruct Res 69:180–190
Hennig W (1981) Insect phylogeny. Wiley, New York
Hopkin SP (1997) Biology of the Springtails. Insecta: Collembola. Oxford University Press, Oxford
Jamieson BGM (1987) The ultrastructure and phylogeny of insect spermatozoa. Cambridge University Press, Cambridge
Jamieson BGM, Dallai R, Afzelius BA (1999) Insects. Their spermatozoa and phylogeny. Scientific Publishers, New Hampshire
Jordan IK, Wolf YI, Koonin EV (2003) No simple dependence between protein evolution rate and the number of protein-protein interactions: only the most prolific interactors tend to evolve slowly. BMC Evol Biol 3:1
Kikushima K (2009) Central pair apparatus enhances outer-arm dynein activities through regulation of inner-arm dynein. Cell Motil Cytoskeleton 66(5):272–280
Kjer KM (2004) Aligned 18S and insect phylogeny. Syst Biol 53:506–514
Kjer KM, Carle FL, Litman J, Ware J (2006) A molecular phylogeny of Insecta. Arthropod Syst Phylog 64:35–44
Klann A, Bird T, Peretti AV, Gromov AV, Alberti G (2009) Ultrastructure of spermatozoa of Solifuges (Arachnida, Solifugae): possible characters for their phylogeny? Tissue Cell 41:91–103
Klass KD, Kristensen NP (2001) The ground plan and affinities of hexapods: recent progress and open problems. Ann Soc Entomol France (NS.) 37:265–298
Koch M (1997) Monophyly and phylogenetic position of the Diplura (Hexapoda). Pedobiology 41:9–12
Kraus O (1997) Phylogenetic relationships between higher taxa of tracheate arthropods. In: Fortey RA, Thomas RH (eds), Arthropod relationships. Systematic association special volume series 55. Chapman and Hall, London, pp 295–303
Kristensen NP (1981) Phylogeny of insect orders. Annu Revue Entomol 26:135–157
Kristensen NP (1998) The groundplain and basal diversification of the hexapods. In: Fortey RA, Thomas RH (eds) Arthropod relationships, systematic association, Ser 55. Chapman and Hall, London, pp 282–293
Kukalova-Peck J (1987) New Carboniferous Diplura, Monura and Thysanura, the hexapod groundplain, and the role of thoracic side lobes in the origin of wings (Insecta). Canad J Zool 65:2327–2345
Lavrov D, Brown WM, Boore JL (2004) Phylogenetic position of the Pentastomida and (pan)crustacean relationships. Proc R Soc London B271:537–544
Luan Y, Mallatt JM, Xie R, Yanng Y, Yin WY (2005) The phylogenetic position of three basal-hexapod groups (Protura, Diplura and Collembola) based on ribosomal RNA gene sequences. Mol Biol Evol 22:1579–1592
Machida R (2006) Evidence from embryology for reconstructing the relationships of Hexapod basal clades. Arthropod Syst Phylog 64:95–104
Mallat J, Giribet G (2006) Further use of nearly complete 28S and 18S rRNA genes to classify Ecdysozoa: 37 more arthropods and a kinorhynch. Mol Phylog Evol 40:772–794
Mencarelli C, Lupetti P, Dallai R (2008) New insights into the cell biology of insect axonemes. Int Rev Cell Mol Biol 268:95–145
Mercati D, Giusti F, Dallai R (2009) A novel membrane specialization in the sperm tail of bug insects (Heteroptera). J Morphol 270:825–833
Michalik P (2006) Zur Morphologie des männlichen Genitalsystems von Spinnen (Araneae, Arachnida) unter besonder Berücksichtigung der Ultrastruktur der Spermien und deren Genese (PhD thesis)
Morrow EH (2004) How the sperm lost its tail: the evolution of aflagellate sperm. Biol Rev 79:795–814
Nardi F, Spinsanti G, Boore JL, Carapelli A, Dallai R, Frati F (2003) Hexapod origins: monophyletic or paraphyletic? Science 299:1887–1889
Negrisolo E, Minelli A, Valle G (2004) The mitochondrial genome of the house centipede Scutigera and the monophyly versus paraphyly of myriapods. Mol Biol Evol 21:770–780
Nosek J (1973) The European Protura. Mus Hist Nat Genève, 346 pp
Paclt J (1956) Biologie der primär flügellosen Insekten. Veb Gustav Fischer Verlag–Jena
Pan J, Snell WJ (2007) The primary cilium: keeper of the key to cell division. Cell 29:1255–1257
Parker GA (1982) Why are there so many tiny sperm? Sperm competition and the maintenance of two sexes. J Theor Biol 96:281–294
Phillips DM (1967) Giant centriole formation in Sciara. J Cell Biol 33:73–92
Pisani D, Polig LL, Lyons-Weiler M, Hedges SB (2004) The colonization of land animals: molecular phylogeny and divergence times among arthropods. BMC Biol 2:1
Porter ME, Sale WS (2000) The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol 151:F37–F42
Proctor HC (1998) Indirect sperm transfer in Arthropods: behavioral and evolutionary trends. Annu Rev Entomol 43:153–174
Quarmby LM, Parker JD (2005) Cilia and the cell cycle? J Cell Biol 6:707–710
Regier JC, Shultz JW, Kambic RE (2004) Phylogeny of basal hexapods lineages and estimates of divergence times. Ann Ent Soc Amer 97:411–419
Regier JC, Shultz JW, Ganley ARD, Hussey A, Shi D, Ball B, Zwick A, Stajich JE, Cummings MP, Martin JW, Cunningham CW (2008) Resolving arthropod phylogeny: exploring signal within 41 kb of protein-coding nuclear gene sequence. Syst Biol 57(6):920–938
Riparbelli MG, Callaini G (2003) Drosophila parthenogenesis: a model for de novo centrosome assembly. Devel Biol 260:298–313
Riparbelli MG, Callaini G, Mercati D, Hertel H, Dallai R (2009) Centrioles to basal bodies in the spermiogenesis of Mastotermes darwiniensis (Insecta, Isopoda). Cell Motil Cytoskel 66:1100–1105
Roosen-Runge EC (1977) The process of spermatogenesis in animals. Cambridge Press, Cambridge
Schaller F (1979) Significance of sperm transfer and formation of spermatophores in arthropod phylogeny. In: Gupta AP (ed) Arthropod phylogeny. Van Nostrand Reihold Co, New York, pp 587–608
Shaller F (1952) Die “Copula” der Collembolen. Naturwissenschaften 39:48
Simmons LW, Siva-Jothy MT (1998) Sperm competition in Insects: mechanism and the potential for selection. In: Birkhead TR, Møller AP (eds) Sperm competition and sexual selection. Academic Press, San Diego, pp 341–434
Smith EF, Sale WS (1992) Regulation of dynein driven microtubule sliding by the radial spokes in flagella. Science 257:1557–1559
Smith EF, Yang P (2004) The radial spokes and central apparatus: mechano-chemical sensors for modulating ciliary and flagellar motility. Cell Motil Cytoskeleton 57:8–17
Spears T, Abele LG (1998) Crustacean phylogeny inferred from 18SrDNA. In: Fortey RA, Thomas RH (eds) Arthropod relationships. Chapman and Hall, London, pp 169–187
Storch V, Jamieson BGM (1992) Further spermatological evidence for including the Pentastomida (tongue worms) in the Crustacea. Int J Parasitol 22:95–108
Štys P, Bilinski S (1990) Ovariole types and the phylogeny of hexapods. Biol Rev 65:401–429
Szöllösi A (1982) Relationships between germ and somatic cells in the testes of locusts and moths. In: King RC, Akai H (eds) Insect ultrastructure, vol 1. Plenum Press, New York and London, pp 32–60
Tuxen SL (1964) The Protura. Hermann, Paris, p 360
Wheeler WC, Whiting M, Wheeler QD, Carpenter JM (2001) The phylogeny of the extant hexapod orders. Cladistics 17:113–169
Willmann R (2003) Phylogenese und system der Insekten. - S. 1-70. In: Lehrbuch der Speziellen Zoologie Bd. I: Wirbellose Tiere, 5. Teil: Insecta (H. H. Dathe, Hrsg.). Spektrum Akademischer Verlag Heidelberg, Berlin
Wingstrand KG (1972) Comparative spermatology of a pentastomid, Raillietiella hemidactyli, and a branchiuran crustacean, Argulus foliaceus, with a discussion of Pentastomid relationships. Munksgaard, Copenhagen, XXIII, pp 1–72
Witte H, Döring D (1999) Canalized pathways of change and constraints in the evolution of reproductive modes of microarthropods. Exp Appl Acarol 23:181–216
Yin WY, Yang Y, Xué L, Dallai R (1985) A “13 + 0” axonemal pattern in the spermatozoon of Neocondeellum dolichotarsum (Insecta, Protura). J Ultrastruct Res 93:179–185
Yin WY, Dallai R, Xué L (1989) Sperm evolution in Protura. In: Dallai R (ed) 3rd International seminar on Apterygota. University of Siena, Siena, pp 195–198
Zrzavý J, Hypša V, Tiez DF (2001) Myzostomida are not Annelids: molecular and morphological support for a clade of animals with anterior sperm flagella. Cladistics 17:170–198
Acknowledgments
We thank Dr. E. Malatesta for technical assistance and Dr E. Swanson for linguistic revision. This work was performed with a grant by Ministry of University and Research (MUR) to R.D. We also thank three anonymous Reviewers for their comments which have improved the paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dallai, R., Mercati, D., Bu, Y. et al. The spermatogenesis and sperm structure of Acerentomon microrhinus (Protura, Hexapoda) with considerations on the phylogenetic position of the taxon. Zoomorphology 129, 61–80 (2010). https://doi.org/10.1007/s00435-009-0100-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-009-0100-1