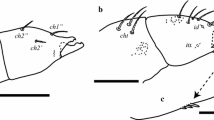
Abstract
The systematic position of Orbiniidae within Polychaeta is still uncertain. In order to provide additional comparative data, we investigated the chaetal arrangement in this family, which is considered valuable for polychaete systematics. Specimens of Scoloplos armiger, Orbinia latreillii, and Pettibonella multiuncinata were examined by SEM and serial sections analysed by computer aided 3D-reconstructions. The obtained data suggest that the chaetal arrangement of Orbiniidae resembles that of other sedentary polychaetes in only a few respects. Transverse rows are only present in the main, anterior part of the chaetal patches of thoracic neuropods. The position of the formative site indicates homology with the transverse rows of several sedentary polychaete taxa. The chaetal patches thus differ significantly from those known in Apistobranchidae. Independent rows with an own caudal formative site, which run along the caudoventral edge of the chaetal patches, resemble the neuropodial ventral longitudinal rows known in Spionidae and related taxa. The abdominal neuropodia of S. armiger and O. latreillii bear longitudinal rows of chaetae. These are reorientated during ontogenetic chaetiger transformation and become the transverse rows of the thoracic chaetal patches. 3D reconstruction of S. armiger revealed that the notopodial chaetal bundles are organized in rows as well. Notopodia and abdominal neuropodia bear deep reaching supportive chaetae. They are the first chaetae formed during neuropodial development and reside dorsally to the longitudinal row of capillary chaetae. Neither position nor structure indicates homology with the supportive chaetae of other sedentary polychaetes. Spionidae and related taxa are thus the only sedentary polychaetes, which specifically resemble Orbiniidae in certain aspects of their chaetal arrangement.





Similar content being viewed by others
References
Bartolomaeus T (1995) Structure and formation of the uncini in Pectinaria koreni, Pectinaria auricoma (Terebellida) and Spirorbis spirorbis (Sabellida): implications for annelid phylogeny and the position of the Pogonophora. Zoomorphology 115:161–177
Bartolomaeus T (1998) Chaetogenesis in polychaetous Annelida—significance for annelid systematics and the position of the Pogonophora. Zool (Jena) 100(4):348–364
Bartolomaeus T, Meyer K (1997) Development and phylogenetic significance of hooked setae in Arenicolidae (Polychaeta, Annelida). Invertebr Biol 116(3):227–242
Bartolomaeus T, Purschke G, Hausen H (2005) Polychaete phylogeny based on morphological data—a comparison of current attempts. Hydrobiologia 535/536:341–356
Blake JA (1975) The larval development of Polychaeta from the northern California coast. III. Eighteen species of Errantia. Ophelia 14:23–84
Blake JA (1996) Family Orbiniidae Hartman, 1942. In: Blake JA, Hilbig B, Scott PH (eds) The Annelida part 3—polychaete: Orbiniidae to Cossuridae. Taxonomic Atlas of the benthic fauna of the Santa Maria Basin and the western Santa Barbara Channel, vol 6. Santa Barbara Museum of Natural History, Santa Barbara, pp 1–26
Blake JA (2000) A new genus and species of polychaete worm (Family Orbiniidae) from methane seeps in the Gulf of Mexico, with a review of the systematics and phylogenetic interrelationships of the genera of Orbiniidae. Cah Biol Mar 41(4):435–449
Bleidorn C (2005) Phylogenetic relationships and evolution of Orbiniidae (Annelida, Polychaeta) based on molecular data. Zool J Linn Soc 144(1):59–73
Bleidorn C, Hausen H (2006) Significance of chaetal arrangement for maldanid systematics (Annelida: Maldanidae). Sci Mar 70(Suppl 3):75–79
Bleidorn C, Vogt L, Bartolomaeus T (2003) New insights into polychaete phylogeny (Annelida) inferred from 18S rDNA sequences. Mol Phylogenet Evol 29:279–288
Blumer MJF, Gahleitner P, Narzt T, Handl C, Ruthensteiner B (2002) Ribbons of semithin sections: an advanced method with a new type of diamond knife. J Neurosci Methods 120:11–16
Eibye-Jacobsen D, Oug E (2002) A report of Hartmaniellidae (Annelida: Polychaeta) from the Andaman Sea, Indian Ocean. In: Eibye-Jacobsen D (ed) Proceedings of the international workshop on the Polychaetes of the Andaman Sea. Phuket Marine Biological Center, Department of Fisheries, Thailand, 3 June–27 August 1997. Phuket Marine Biological Center Special Publication 24:141–147
Eisig J (1914) Zur Systematik, Anatomie und Morphologie der Ariciiden nebst Beiträgen zur generellen Systematik. Mitteil Zool Stat Neapel 21(6):153–600
Gibbs PE (1968) Observations on the population of Scoloplos armiger at Whitstable. J Mar Biol Assoc UK 48:225–254
Halanych KM, Janosik AM (2006) A review of molecular markers used for Annelid phylogenetics. Integr Comp Biol 46(4):533–543
Hall KA, Hutchings P, Colgan D (2004) Further phylogenetic studies of the Polychaeta using 18S rDNA sequence data. J Mar Biol Assoc UK 84:949–960
Hartman O (1957) Orbinidae, Apistobranchidae, Paraonidae and Longosomidae. In: Allan Hancock Pacific Expeditions 15. University of Southern California Press, Los Angeles, California, pp 211–393, pl. 20–45
Hartman O, Barnard JL (1958) The benthic fauna of the deep basins off southern California. Allan Hancock Pacific Expeditions 22(1):1–67, pl. 1–2. University of Southern California Press, Los Angeles, California
Hartmann-Schröder G (1996) Annelida, Borstenwürmer, Polychaeta. Die Tierwelt Deutschlands, vol 58. Gustav Fischer, Jena
Hausam B, Bartolomaeus T (2001) Ultrastructure and development of forked and capillary setae in the polychaetes Orbinia bioreti and Orbinia latreillii (Annelida: Orbiniidae). Invertebr Biol 120(1):13–28
Hausen H (2005) Chaetae and chaetogenesis in polychaetes (Annelida). Hydrobiologia 535/536:37–52
Hausen H, Bartolomaeus T (1998) Setal structure and chaetogenesis in Scolelepis squamata and Malacoceros fuliginosus (Spionidae, Annelida). Acta Zool 79(3):149–161
Kristensen RM, Nørrevang A (1982) Description of Psammodrilus aedificator sp.n. (Polychaeta), with notes on the Arctic interstitial fauna of Diso Island, W. Greenland. Zool Scr 11(4):265–279
Mackie ASY (1984) On the identity and zoogeography of Prionospio cirrifera Wirén, 1883 and Prionospio multibranchiata Berkeley, 1927 (Polychaetae: Spionidae). In: Hutchings PA (ed) Proceedings of the 1st international polychaete conference. The Linnean Society of New South Wales, Sydney, pp 35–47
Mackie ASY (1987) A review of species currently assigned to the genus Leitoscoloplos Day, 1977 (Polychaeta: Orbiniidae), with descriptions of species newly referred to Scoloplos Blainville, 1828. Sarsia 72:1–28
Mackie ASY (1990) Prionospio saccifera sp. nov. (Polychaeta: Spionidae) from Hong Kong and the Red Sea, with a redescription of Prionospio ehlersi Fauvel, 1928. In: Morton B (ed) Proceedings of the 2nd international marine biological workshop: The Marine Flora and Fauna of Hong Kong and Southern China, Hong Kong, 1986. Hong Kong University Press, Hong Kong, pp 363–375
Meyer K, Bartolomaeus T (1996) Ultrastructure and formation of the hooked setae in Owenia fusiformis delle Chiaje, 1842: implications for annelid phylogeny. Can J Zool 74:2143–2153
O’Clair RM, Cloney RA (1974) Patterns of morphogenesis mediated by dynamic microvilli: Chaetogenesis in Nereis vexillosa. Cell Tiss Res 151:141–157
Orrhage L (1974) Über die Anatomie, Histologie und Verwandtschaft der Apistobranchidae (Polychaeta Sedentaria) nebst Bemerkungen über die systematische Stellung der Archianneliden. Z Morphol Tiere 79:1–45
Pernet B, Qian P-Y, Rouse G, Young CM, Eckelbarger KJ (2002) Phylum Annelida. In: Young CM, Sewell MA Rice ME (eds) Atlas of marine invertebrate larvae. Academic, San Diego, pp 209–243
Planas M, Mora J (1989) Epigenetical changes in Capitella (Polychaeta, Capitellidae) in the Ensenada de Lourizan (NW Spain). Vie Milieu Paris 39(3–4):159–163
Purschke G, Hausen H (2007) Lateral organs in sedentary polychaetes (Annelida) – Ultrastructure and phylogenetic significance of an insufficiently known sense organ. Acta Zool 88:23–39
Radashevsky VI, Fauchald K (2000) Chaetal arrangement and homology in spionids (Polychaeta: Spionidae). Bull Mar Sci 67(1):13–23
Rouse GW, Fauchald K (1997) Cladistics and polychaetes. Zool Scr 26(2):139–204
Rousset V, Pleijel F, Rouse GW, Siddal ME (2007) A molecular phylogeny of annelids. Cladistics 23(1):41–63
Schweigkofler M, Bartolomaeus T, von Salvini-Plaven L (1998) Ultrastructure and formation of hooded hooks in Capitella capitata (Fabricius, 1780) (Capitellida, Annelida). Zoomorphology 118:117–128
Solis-Weiss V, Fauchald K (1989) Orbiniidae (Annelida: Polychaeta) from mangrove root-mats in Belize, with a revision of protoariciin genera. Proc Biol Soc Wash 102(3):772–792
Storch V, Schlötzer-Schrehardt U (1988) Sensory structures. In: Westheide W, Hermans CO (eds) The ultrastructure of Polychaeta. Mikrofauna Mar 4:121–133
Struck TH, Schult N, Kusen T, Hickman E, Bleidorn C, McHugh D, Halanych KM (2007) Annelid phylogeny and the status of Sipuncula and Echiura. BMC Evol Biol 7:57–67
Wilson DP (1929) The larvae of the British sabellarians. J Mar Biol Assoc UK 16:221–269
Acknowledgments
Our thanks are due to C. Bleidorn, who gave helpful remarks on orbiniid ingroup relationship, T. Bartolomaeus for support and discussion and L. Vogt, D. Eibye-Jacobsen and two anonymous reviewers, whose comments improved the manuscript. S. Albrecht gave worthful hints for sampling juvenile S. armiger. This work was financially supported by DFG (HA 443/2-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Dr. Wilfried Westheide on the occasion of his 70th birthday.
Rights and permissions
About this article
Cite this article
Hoffmann, S., Hausen, H. Chaetal arrangement in Orbiniidae (Annelida, Polychaeta) and its significance for systematics. Zoomorphology 126, 215–227 (2007). https://doi.org/10.1007/s00435-007-0042-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-007-0042-4