Abstract
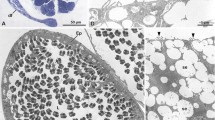
The acrosome-less spermatozoon of Aristaeopsis edwardsiana (Crustacea, Aristeidae), which consists of a central non-membrane bound nuclear region surrounded by a thin peripheral cytoplasm, much resembles that of the previously studied aristeid Aristaeomorpha foliacea. The marked spermatozoal similarities between these two species appear to indicate a close phylogenetic proximity. A considerably different spermatozoal pattern is observed in aristeids from the genus Aristeus (A. varidens and A. antennatus), whose spermatozoa possess an anterior spherical acrosome, lacking a spike, and partially embedded in (instead of capping) the main sperm body. The two distinct sperm types found in the Aristeidae differ significantly from the spiked sperm typically found in most penaeoids (Penaeidae, Solenoceridae and Sicyoniidae), thus suggesting a phylogenetic separation of the Aristeidae from the remaining Penaeoidea.



Similar content being viewed by others
References
Brown GG (1966) Ultrastructural studies of sperm morphology and sperm–egg interaction in the decapod Callinectes sapidus. J Ultrastruct Res 14:425–440
Clark WH Jr, Griffin FJ (1988) The morphology and physiology of the acrosome reaction in the sperm of the decapod, Sicyonia ingentis. Dev Growth Differ 30:451–462
Clark WH Jr, Kleve MG, Yudin AI (1981) An acrosome reaction in natantian sperm. J Exp Zool 218:279–291
Demestre M, Fortuño J-M (1992) Reproduction of the deep-water shrimp Aristeus antennatus (Decapoda, Dendrobranchiata). Mar Ecol Prog Ser 84:41–51
Demestre M, Cortadellas N, Durfort M (1993) Ultrastructura de les espermatides de la gamba, Aristeus antennatus (Crustaci, Decapoda). Biologia de la Reproducció 3:14–17
Demestre M, Cortadellas N, Durfort M (1997) Ultrastructure of the sperm of the deep-sea decapod Aristeus antennatus. J Morphol 234:79–87
Dougherty WJ, Dougherty MM (1989) Electron microscopical and histochemical observations on melanized sperm and spermatophores of pond-cultured shrimp, Penaeus vannamei. J Invert Pathol 54:331–343
Felgenhauer BE, Abele LG (1991) Morphological diversity of decapod spermatozoa. In: Bauer RT, Martin JW (eds) Crustacean sexual biology. Columbia University Press, New York, NY, pp 322–341
Goudeau M (1982) Fertilization in a crab: I. Early events in the ovary, and cytological aspects of the acrosome reaction and gamete contacts. Tissue Cell 14:97–111
Griffin FJ, Clark WH Jr (1990) Induction of acrosomal filament formation in the sperm of Sicyonia ingentis. J Exp Zool 254:296–304
Griffin FJ, Shigekawa K, Clark WH Jr (1988) Formation and structure of the acrosomal filament in the sperm of Sicyonia ingentis. J Exp Zool 246:94–102
Hertzler PL, Clark WH Jr (1993) The late events of fertilisation in the penaeoidean shrimp Sicyonia ingentis. Zygote 1:287–296
Hinsch GW (1971) Penetration of the oocyte envelope by spermatozoa in the spider crab. J Ultrastruct Res 35:86–97
Jamieson BGM (1991) Ultrastructure and phylogeny of crustacean spermatozoa. Mem Queensl Mus 31:109–142
Jamieson BGM, Tudge CC (2000) Crustacea-Decapoda. In: Adiyodi KG, Adiyodi RG, Jamieson BGM (eds) Reproductive biology of invertebrates. Progress in male gamete ultrastructure and phylogeny, vol 9C. Wiley, Chichester, pp 1–95
Jamieson BGM, Ausio J, Justine J-L (eds) (1995a) Advances in spermatozoal phylogeny and taxonomy. Mémoires du Muséum National d’Histoire Naturelle Paris 166:343–358
Jamieson BGM, Guinot D, Richer de Forges B (1995b) Phylogeny of the Brachyura (Crustacea, Decapoda): evidence from spermatozoal ultrastructure. In: Jamieson BGM, Ausio J, Justine J-L (eds) Advances in spermatozoal phylogeny and taxonomy. Mémoires du Muséum National d’Histoire Naturelle Paris 166:265–283
Kleve MG, Yudin AI, Clark WH Jr (1980) Fine structure of the unistellate sperm of the shrimp, Sicyonia ingentis (Natantia). Tissue Cell 12:29–45
Krol RM, Hawkins WE, Overstreet RM (1992) Reproductive components. In: Harrison FW, Humes AG (eds) Microscopic anatomy of invertebrates. Decapod Crustacea, vol 10. Wiley-Liss, New York, NY, pp295–343
Martin JW, Davis GE (2001) An updated classification of the recent Crustacea. Science Series No. 39. Natural History Museum of Los Angeles County
Medina A (1992) Structural modifications of sperm from the fiddler crab Uca tangeri (Decapoda) during early stages of fertilization. J Crustac Biol 12:610–614
Medina A (1994) Spermiogenesis and sperm structure in the shrimp Parapenaeus longirostris (Crustacea, Dendrobranchiata): comparative aspects among decapods. Mar Biol 119:449–460
Medina A (1995a) Spermatozoal ultrastructure in Dendrobranchiata (Crustacea, Decapoda): taxonomic and phylogenetic considerations. In: Jamieson BGM, Ausio J, Justine J-L (eds) Advances in spermatozoal phylogeny and taxonomy. Mémoires du Muséum National d’Histoire Naturelle Paris 166:231–242
Medina A (1995b) The atypical sperm morphologies of Aristeus antennatus and Aristaeomorpha foliacea (Crustacea, Dendrobranchiata, Aristeidae) and their phylogenetic significance. In: Jamieson BGM, Ausio J, Justine J-L (eds) Advances in spermatozoal phylogeny and taxonomy. Mémoires du Muséum National d’Histoire Naturelle Paris 166:243–250
Medina A, Rodríguez A (1992) Structural changes in sperm from the fiddler crab, Uca tangeri (Crustacea, Brachyura), during the acrosome reaction. Mol Reprod Dev 33:195–201
Medina A, López de la Rosa I, Santos A (1994a) Ultrastructural comparison of the spermatozoa of Sicyonia carinata (Sicyoniidae) and Penaeopsis serrata (Penaeidae) shrimps (Crustacea, Dendrobranchiata), with particular emphasis on the acrosomal structure. J Submicrosc Cytol Pathol 26:395–403
Medina A, Mourente G, López de la Rosa I, Santos A, Rodríguez A (1994b) Spermatozoal ultrastructure of Penaeus kerathurus and Penaeus japonicus (Crustacea, Dendrobranchiata). Zoomorphology 114:161–167
Medina A, Vila Y, Santos A (1998) The sperm morphology of the euphausiid Meganyctiphanes norvegica (Crustacea, Eucarida). Invert Reprod Dev 34:65–68
Medina A, Scelzo MA, Tudge CC (2005) Spermatozoal ultrastructure in three Atlantic solenocerid shrimps (Decapoda, Dendrobranchiata). J Morphol (in press)
Pérez-Farfante I, Kensley B (1997) Penaeoid and sergestoid shrimps and prawns of the world. Mémoires du Muséum National d’Histoire Naturelle Paris 175:233
Pochon-Masson J (1968) L’ultrastructure des spermatozoïdes vésiculaires chez les crustacés décapodes avant et au cours de leur dévagination expérimentale. I. Brachyoures et Anomoures. Ann Sci Nat (Zool) 10:1–100
Scelzo MA, Medina A (2003) Spermatozoal ultrastructure in Artemesia longinaris (Decapoda, Penaeidae). J Crust Biol 23:814–818
Scelzo MA, Medina A (2004) A dendrobranchiate, Peisos petrunkevitchi (Decapoda, Sergestidae), with reptant-like sperm: a spermiocladistic assessment. Acta Zool 85:81–89
Talbot P, Chanmanon P (1980) Morphological features of the acrosome reaction of lobster (Homarus) sperm and the role of the reaction in generating forward sperm movements. J Ultrastruct Res 70:287–297
Talbot P, Summers RG (1978) The structure of sperm from Panulirus, the spiny lobster, with special regard to the acrosome. J Ultrastruct Res 64:341–351
Talbot P, Poolsanguan B, Al-Hajj H, Poolsanguan W (1991) Gamete interactions during in vitro fertilization of lobster (Homarus americanus) oocytes. J Struct Biol 106:125–134
Tudge CC (1995) Ultrastructure and phylogeny of the spermatozoa of the infraorders Thalassinidea and Anomura (Decapoda, Crustacea). In: Jamieson BGM, Ausio J, Justine J-L (eds) Advances in spermatozoal phylogeny and taxonomy. Mémoires du Muséum National d’Histoire Naturelle Paris 166:251–263
Tudge CC, Scheltinga DM, Jamieson BGM (1998) Spermatozoal ultrastructure in the spiny lobster Jasus novaehollandiae Holthuis, 1963 (Palinuridae, Palinura, Decapoda). J Morphol 236:117–126
Yudin AI, Clark WH Jr, Kleve MG (1979) An acrosome reaction in natantian sperm. J Exp Zool 210:569–574
Acknowledgements
The invaluable technical assistance of Agustín Santos (Biology Department) and the staff of the Electron Microscopy Unit (University of Cádiz) are greatly appreciated. We thank two anonymous reviewers for helpful comments and recommendations. This study was partially supported by the Spanish government (project REN2001-2875/MAR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Medina, A., García-Isarch, E., Sobrino, I. et al. Ultrastructure of the spermatozoa of Aristaeopsis edwardsiana and Aristeus varidens (Crustacea, Dendrobranchiata, Aristeidae). Zoomorphology 125, 39–46 (2006). https://doi.org/10.1007/s00435-005-0013-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00435-005-0013-6