Abstract
Introduction
Long-term survivors have an increased risk of developing secondary solid malignancies (SSMs) after allogeneic-hematopoietic stem cell transplantation (allo-HSCT) with graft-versus-host disease (GVHD) potentially modulating these risks.
Methods
This retrospective study analyzed the cumulative incidences of SSMs after chemotherapy-based conditioning for allo-HSCT patients with acute myeloid leukemia (n = 266) transplanted at the University Hospital Regensburg between 1999 and 2016.
Results
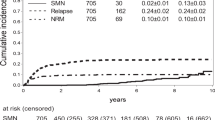
The median follow-up was 11.4 years (Interquartile range, 9.0–14.9). The 100-day cumulative incidence of grade II-IV acute GVHD (aGVHD) was 44.4% [95% CI (38.3, 50.2)], while the 5-year cumulative incidence of chronic GVHD (cGVHD, requiring systemic immunosuppression) was 36.9% [95% CI (31.1, 42.6)]. The cumulative incidences of secondary squamous cell carcinomas (SCCs) at 10 and 15 years were 4.2% [95% CI (2.2, 7.2)] and 8.1% [95% CI (4.6, 12.8)], while the cumulative incidences of non-SCCs at 10 and 15 years were 5.4% [95% CI (3.1, 8.7)] and 6.9% [95% CI (4.0, 10.8)]. Antithymocyte globulin (ATG) was associated with reduced incidences of SCCs but not of non-SCCs. Patients with grade II-IV aGVHD had increased rates of SCCs after adjusting with patient age and ATG, while patients with cGVHD showed only a trend for increased rates of SCCs.
Conclusion
The data indicate that aGVHD and cGVHD affect the rates of secondary SCCs. While the use of ATG is associated with lower incidences of SCCs via reduction of GVHD, there was no association of ATG with non-SCCs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is a curative treatment option for selected patients with acute myeloid leukemia (AML). However, allo-HSCT is associated with a relatively high long-term non-relapse mortality (NRM), including secondary solid malignancies (SSMs) (Curtis et al. 1997). Factors modifying the risks of SSMs are the primary diagnosis, genetic predisposition, patient age, conditioning regimens with either total body irradiation (TBI) or chemotherapy alone, infections with oncogenic viruses and graft-versus-host disease (GVHD) (Curtis et al. 1997; Miller and Johnstone 2001; Rizzo et al. 2009). Chronic GVHD (cGVHD) occurs in approximately 50% of patients and represents the main cause of long-term morbidity and mortality after allo-HSCT with acute GVHD (aGVHD) known to be the main risk factor (Lee et al. 2003; Stewart et al. 2004; Grube et al. 2016). It is still unclear how GVHD alters the risks of SSMs and whether non-squamous cell carcinomas (non-SCCs) and SCCs are similarly influenced by GVHD. Unfortunately, literature focusing on SSMs after allo-HSCT shows heterogeneity in conditioning regimens (TBI-based, chemotherapy-only), patient population (leukemia, lymphoma or aplastic anemia) and types of SSMs reported with little information on individual cancer types. In this retrospective study, we, therefore, analyzed the cumulative incidences of SSMs and precancerous lesions after non-TBI-based conditioning focusing on AML patients with the provision of details of all SSMs. The objective of this study was to analyze the effects of pre-transplantation variables on the cumulative incidences of SSMs and to estimate the effects of aGVHD and cGVHD on the rates for SSMs.
Data collection
We retrospectively analyzed the cumulative incidences of SSMs, precancerous lesions (carcinomas in situ, actinic keratosis of the skin) and histologically proven atypical nevi after non-TBI-based conditioning in AML patients who received their 1st allo-HSCT at the Department of Hematology of the University Hospital Regensburg between 1999 and 2016. Post-transplant lymphoproliferative disorders were not analyzed. Secondary solid malignancies were subdivided into invasive squamous cell carcinomas (SCCs) and non-squamous cell carcinomas (non-SCCs). Eligibility criteria for this retrospective analysis included adult patients with primary or secondary AML (treatment-related AML) who underwent their 1st allo-HSCT from matched sibling donors (MSD), matched unrelated donors (MUD), mismatched unrelated donors (MMUD) or haploidentical/mismatched related donors (MMRD) after non-TBI-based conditioning. The choice of conditioning regimens and GVHD prophylaxis was based on the oncologists´ discretion and dependent on the patient´s age, disease risk, comorbidities and donor type (antithymocyte globulin was standard in unrelated donor transplantation and at the discretion of the physicians in sibling donor transplantation). As TBI-based patients are younger, we didn’t analyze TBI-patients to prevent an age bias. Clinical data were extracted from the medical charts of the Department of Hematology, University Hospital Regensburg. Transplantation variables included patient age at the time of allo-HSCT, sex, diagnosis, Karnofsky performance score (KPS), hematopoietic cell transplantation-comorbidity index (HCT-CI) as described by Sorror et al. (2005), 2017 European LeukemiaNet (ELN) genetic risk stratification as described by Döhner et al. (2017), disease status before allo-HSCT, stem cell source, conditioning regimens, recipient and donor characteristics (donor age, HLA-compatibility, gender match, cytomegalovirus serostatus), GVHD prophylaxis and the use of antithymocyte globulin (ATG). We captured grade II-IV aGVHD, cGVHD requiring systemic immunosuppressive therapy, the duration of systemic immunosuppressive therapy of cGVHD and sites of cGVHD (skin, oral mucosa, liver, lung, eyes, gastrointestinal tract, joints, fascia, genitals and cGVHD of the central nervous system). All patients received screenings for cutaneous malignancies before allo-HSCT. The screening program of SSMs after allo-HSCT included physical examinations including examinations of the skin, thyroid glands, oral cavity and pharynx during annual control. Patients at high risk for developing oropharyngeal cancer were screened every 6 months (e. g., in cases of GVHD). Colorectal, gynecological and urological screening was recommended once a year. Data closing was in October 2023. The local Ethics Board of the University of Regensburg approved this study (number, 20-1810-101).
Definitions and statistical endpoints
The primary endpoints were the cumulative incidences of SSMs with deaths without prior SSMs considered as competing events. SSMs were subdivided into SCCs and non-SCCs. A separate analysis comprised precancerous lesions (carcinomas in situ, actinic keratosis of the skin) and histologically proven atypical nevi. For patients developing more than one SSM or precancerous lesions, the time to the first lesion was recorded. Acute GVHD was classified as clinically significant at grade II–IV aGVHD. Acute GVHD and cGVHD were defined according to described standard criteria (Filipovich et al. 2005; Jagasia et al. 2015). The cumulative incidences of grade II-IV aGVHD were estimated considering death or relapse without grade II-IV aGVHD as competing events. For the cumulative incidences of cGVHD requiring systemic immunosuppressive treatment, relapse or death without prior cGVHD (requiring systemic immunosuppressive treatment) was counted as a competing event. Additionally, we analyzed the effects of pre-transplantation variables on the incidences of SSMs (SCCs and non-SCCs) using a multivariable Fine-Gray regression model accounting for the respective competing events (death without prior SSM). Pre-transplantation covariates were patient age, conditioning regimens, donor type, graft source, sex match, donor age, donor/recipient CMV serostatus and the use of ATG. The impact of time-varying variables (aGVHD and cGVHD) on the rates of SSMs was analyzed using cause-specific hazard (CSH) models in a counting process format with adjustment of patient age and ATG. In cases with ongoing systemic immunosuppressive therapy at the time of diagnosis of cGVHD (e. g., therapy of aGVHD), we recorded the duration of the entire immunosuppressive therapy for GVHD. First-line treatment of cGVHD consisted of corticosteroids given alone or combined with calcineurin or mTOR inhibitors. The choice of second and third-line therapies depended mainly on the comorbidities, the risk profile of cGVHD and patients´ medical history. Information on the last day of systemic immunosuppressive therapy for cGVHD was missing in 6 patients with cGVHD. All times to the endpoints were calculated from the date of allo-HSCT (day 0). If a patient was event-free for all of the endpoints, the patient was censored at the last date of follow-up with confirmation of being event-free.
Statistical analysis
Transplant-related characteristics are presented as median and interquartile range (IQR) for continuous variables and as absolute and relative frequencies for categorical variables. We used cumulative incidence functions (CIF) to describe the incidences of SSMs accounting for the competing risks (death without prior SSMs). Fine and Gray models described the effects of pre-transplantation variables on the subdistribution hazard functions. The proportional hazard assumption of the Fine and Gray models was tested by using Schoenfeld-type residuals. The effects of aGVHD and cGVHD on cause-specific hazards of SSMs were estimated with Cox proportional hazard regression treating all other events as censored. Acute GVHD and cGVHD were analyzed using a counting process format with adjustment of patient age and ATG. Hazard Ratio (HR) and 95% confidence intervals (95% CI) are presented as effect estimates. Median follow-up time was estimated using the reverse Kaplan–Meier method. All P-values were two-sided, and P < 0.05 were considered significant. Statistical analysis was performed using R, version 4.3.2 (R Core Team. R: A language for statistical computing. 2014. The R Foundation for Statistical Computing, Vienna, Austria) and SPSS 26.0 (SPSS Inc., Chicago, IL, USA).
Results
Patient and transplant characteristics
Table 1 summarizes transplant characteristics. Between 1999 and 2016, 266 patients received their 1st allo-HSCT for de novo/primary AML (n = 178) or secondary AML (n = 88) after non-TBI-based conditioning with peripheral blood (n = 244) or bone marrow (n = 22) as a stem cell source. The median patient age at allo-HSCT was 55.9 years (IQR, 45.8–61.4). The median follow-up time was 11.4 years (IQR, 9.0–14.9). All patients received reduced-intensity conditioning regimens (RIC-regimens) with Melphalan-based chemotherapy (n = 193) as the most frequent regimen. Table 2 summarizes all conditioning regimens.
Acute and chronic graft-versus-host disease
The 100-day cumulative incidence of grade II-IV aGVHD was 44.4% [95% CI (38.3, 50.2)], while the 2-year and 5-year cumulative incidences of cGVHD (requiring systemic immunosuppression) were 35.0% [95% CI (29.2, 41.4)] and 36.9% [95% CI (31.1, 42.6)]. Table 3 shows graft-versus-host disease characteristics. The most common sites of cGVHD were skin (77.8%), oral mucosa (67.7%) and eyes (59.6%). Most patients had multi-organ involvement of cGVHD (Table 3). The median time of systemic immunosuppressive therapy of cGVHD was 729.0 days (IQR, 337.5–1715.0) in patients developing cGVHD.
Secondary solid malignancies including precancerous lesions
Table 4 shows all precancerous lesions, atypical nevi (n = 15) and SSMs (n = 42) with the duration of systemic immunosuppression applied for treatment of cGVHD. In summary, 42 SSMs in 32 patients were recorded. The cumulative incidences of any invasive SSMs at 5, 10 and 15 years were 6.0% [95% CI (3.6, 9.3)], 8.6% [95% CI (5.5, 12.4)] and 12.2% [95% CI (8.1, 17.2)], while the cumulative incidences of death (competing risk) at 5, 10 and 15 years were 54.5% [95% CI (48.3, 60.3)], 56.1% [95% CI (49.9, 61.8)] and 58.0% [95% CI (51.3, 64.1)], respectively.
Secondary squamous cell carcinomas
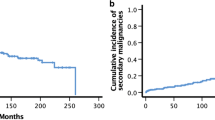
Nineteen invasive SCCs were recorded in 16 patients (Table 4). Fourteen patients developed one SCC, one patient two SCCs and one patient three SCCs. The most common SCCs were cutaneous SCCs (n = 9) and SCCs of the head and neck region (n = 5). The mean time from allo-HSCT to the development of SCCs was 21.3 years [95% CI (20.0, 22.7)]. Figure 1 shows the estimates of the cumulative incidences of secondary SCCs. The cumulative incidences of SCCs at 5, 10 and 15 years were 2.6% [95% CI (1.2, 5.1)], 4.2% [95% CI (2.2, 7.2)] and 8.1% [95% CI (4.6, 12.8)], respectively (Fig. 1). Within this group of SCCs, the cumulative incidences of cutaneous SCCs at 5, 10 and 15 years were 1.1% [95% CI (0.3, 3.1)], 1.1% [95% CI (0.3, 3.1)] and 3.5% [95% CI (1.3, 7.5)].
Secondary non-squamous cell carcinomas
In summary, 23 invasive non-SCCs in 16 patients were recorded (Table 4). Ten patients had one non-SCC, five patients had two non-SCCs and one patient had three non-SCCs. The most common cancer types were cutaneous basal cell carcinomas (BCCs, n = 14) and malignant melanomas (n = 3). The mean time from allo-HSCT to the development of invasive non-SCCs was 21.6 years [95% CI (20.3, 23.0)]. The cumulative incidences of non-SCCs at 5, 10 and 15 years were 3.8% [95% CI (1.9, 6.6)], 5.4% [95% CI (3.1, 8.7)] and 6.9% [95% CI (4.0, 10.8)], respectively (Fig. 2). Within this group of non-SCCs, the cumulative incidences of cutaneous BCCs at 5, 10 and 15 years were 1.9% [95% CI (0.7, 4.1)], 2.8% [95% CI (1.2, 5.5)] and 4.3% [95% CI (2.0, 7.9)].
Precancerous lesions and atypical nevi
Eight cutaneous carcinomas in situ, four histologically proven atypical nevi, one actinic keratosis, one intraepithelial neoplasia of the colon, and one vulvar intraepithelial neoplasia (VIN III) in 11 patients were diagnosed (Table 4). The cumulative incidences of precancerous lesions and atypical nevi at 5, 10 and 15 years were 2.6% [95% CI (1.2, 5.1)], 3.8% [95% CI (2.0, 6.7)] and 4.9% [95% CI (2.4, 8.8)], respectively.
Multivariate analysis of secondary solid malignancies and pre-transplantation variables
Table 5 depicts the multivariate analysis of pre-transplantation variables and SSMs using multivariable Fine and Gray proportional hazards regression models. The use of ATG was associated with reduced incidences of SCCs [HR, 0.09, 95% CI (0.02, 0.40); P = 0.002] compared to patients not receiving ATG. The multivariate analysis found no association of ATG with the incidences of non-SCCs (Table 5).
Cause-specific hazard ratios for development of secondary solid malignancies
Tables 6 and 7 show the cause-specific hazard ratios of aGVHD and cGVHD for SSMs after adjustment of patient age (Model 1) and after adjustment of patient age and ATG (Model 2). Patients with grade II-IV aGVHD had significantly increased rates of SCCs after adjusting with patient age and ATG (Table 6), while patients with cGVHD showed only a trend for increased rates of SCCs (Table 7). GVHD variables did not influence the rates of non-SCCs.
Discussion
This retrospective study analyzed incidences of SSMs including non-melanoma skin cancers after chemotherapy-only conditioning focusing on AML patients and 1st allo-HSCT. In the present study, the overall cumulative incidences of SSMs at 10 and 15 years were 8.6% and 12.2%, similar to recent studies (Martelin et al. 2019; Novitzky-Basso et al. 2020). Novitzky-Basso et al. (2020) revealed overall incidences of SSMs of 19.5% [95% CI (15.9, 23.4)] at 12 years with cutaneous BCCs, cutaneous SCCs and head and neck cancers the most frequent cancer types. Martelin et al. (2019) reported cumulative incidences of SSMs of 13.9% at 15 years (excluding non-melanoma skin cancers). Similar to other studies including non-melanoma skin cancers, cutaneous SCCs and BCCs were the most frequent SSMs in the present study, with cumulative incidences of 3.5% and 4.3% at 15 years. Leisenring et al. (2006) reported 15-year cumulative incidences of cutaneous and mucosal SCCs and BCCs of 2.2% [95% CI (1.7, 2.8)] and 4.0% [95% CI (3.3, 4.8)] in a large cohort of 4,810 patients. Precancerous lesions and atypical nevi were also identified with the help of annual dermatological examinations at the transplant center of Regensburg. The cumulative incidences of precancerous lesions and atypical nevi at 10 and 15 years were 3.8% and 4.9%, respectively. The majority of studies focusing on SSMs did not include non-melanoma skin cancers in the analysis resulting in comparatively low incidences of SSMs. Furthermore, the literature on SSMs shows variability in primary diagnoses (leukemia, lymphoma and aplastic anemia), patient age and conditioning regimens (TBI-based, chemotherapy-only) making a comparison of these results difficult. Acute myeloid leukemia, chronic myeloid leukemia (CML) and acute lymphoblastic leukemia (ALL) seem to have higher risks in comparison to other primary diagnoses (Curtis et al. 1997; Bhatia et al. 2001) contributing to the relatively high cumulative incidences of SSMs of the present study comprising of AML patients.
Chronic GVHD is a main cause of morbidity and mortality after allo-HSCT (Lee et al. 2003). In multivariate analyses, the use of ATG was associated with a lower incidence of secondary SCCs, which may be explained by the reduction in aGVHD and cGVHD (Finke et al. 2009; Kröger et al. 2016). We used a cause-specific hazard model to analyze the effects of aGVHD and cGVHD on the rates of SSMs after adjustment of patient age and ATG. Our results indicate that aGVHD is associated with an increased rate of secondary SCCs after adjusting for patient age and ATG, while cGVHD showed only a trend for an increased rate of SCCs. In summary, the present study confirms the associations between GVHD and secondary SCCs, as indicated by Leisenring et al. (2006) and Curtis et al. (1997). Curtis et al. (1997) concluded that cGVHD is strongly related to the risks for SCCs. This study revealed that patients with cGVHD receiving immunosuppressive therapy for two or more years showed an association with secondary SCCs of the buccal cavity and skin (Curtis et al. 1997). The results of the present study indicate no association of cGVHD with the risks for non-SCC contrary to the results of Leisenring et al. demonstrating a relationship between cGVHD and the risks for non-SCCs (Leisenring et al. 2006). In the present study, four patients developed SCCs of the oral cavity at anatomic sites previously affected by cGVHD, as did the patient with SCC of the esophagus. All patients with malignant melanomas of the skin had a history of cGVHD of the skin. These cases indicate that cGVHD is a relevant risk factor for SSMs of the skin and mucosa (Demarosi et al. 2005). Our results are in line with the review of Demarosi et al. (2005) reporting an elevated risk of SCCs of the oral cavity in patients with cGVHD. Human papillomaviruses (HPV) may further increase the risks of SCCs of the mucosa and skin in patients with systemic immunosuppression (Miller and Johnstone 2001). In the present study, two women with a history of cGVHD developed SCCs of the cervix and vulva both associated with HPV. In summary, the relatively high cumulative incidences of SSMs are not solely based on GVHD but may result from a selection bias, as patients were at relatively old age at the time of allo-HSCT (median age, 55.9 years). This risk-based selection treating older patients with chemotherapy-only conditioning and younger with TBI-based conditioning was defined in institutional guidelines and is in line with recommendations.
The latency period for the development of SSMs is relatively long. Therefore, more SSMs are diagnosed as we obtain longer follow-ups. Literature indicates that GVHD-related SSMs such as SCCs of the skin and oropharynx occur early after allo-HSCT as can be assumed by the cases of the present study. In contrast, the literature indicates that TBI-related SSMs occur with long delay after allo-HSCT (Rizzo et al. 2009). Independent of the conditioning regimens, lifelong screening for SSMs is recommended after allo-HSCT. The screenings for SSMs include skin, thyroid, head and neck and gynecological examinations, as well as assessing symptoms of any kind of SSMs during annual control (Socié and Rizzo 2012). Furthermore, avoidance of additional carcinogenic sources, eg. tobacco, alcohol, and sun exposure are recommended to reduce the risk of SSMs (Socié and Rizzo 2012). This study is limited by its retrospective design and the relatively low number of patients. The primary strength of the present study is the homogeneity of the study population conditioned with non-TBI-based regimens and the details of all SSMs.
Conclusions
Second solid malignancies occur at any site and histology in patients after allo-HSCT with cutaneous SCCs and BCCs having the highest incidences. Data indicate that aGVHD and cGVHD are risk factors for the development of secondary SCCs after allo-HSCT without association with non-SCCs. Furthermore, the incidences of secondary SCCs seem to be reduced by the use of ATG which results in a reduced incidence of cGVHD. Whether other regimens like post-transplant Cyclophosphamide result in the same reduction of SCCs remains to be shown.
Data availability
Datasets generated during the current study are available from the corresponding author on reasonable request.
References
Bhatia S, Louie AD, Bhatia R et al (2001) Solid cancers after bone marrow transplantation. J Clin Oncol off J Am Soc Clin Oncol 19:464–471. https://doi.org/10.1200/JCO.2001.19.2.464
Curtis RE, Rowlings PA, Deeg HJ et al (1997) Solid cancers after bone marrow transplantation. N Engl J Med 336:897–904. https://doi.org/10.1056/NEJM199703273361301
Demarosi F, Lodi G, Carrassi A et al (2005) Oral malignancies following HSCT: graft versus host disease and other risk factors. Oral Oncol 41:865–877. https://doi.org/10.1016/j.oraloncology.2005.02.001
Döhner H, Estey E, Grimwade D et al (2017) Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 129:424–447. https://doi.org/10.1182/blood-2016-08-733196
Filipovich AH, Weisdorf D, Pavletic S et al (2005) National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. diagnosis and staging working group report. Biol Blood Marrow Transplant 11:945–956. https://doi.org/10.1016/J.BBMT.2005.09.004
Finke J, Bethge WA, Schmoor C et al (2009) Standard graft-versus-host disease prophylaxis with or without anti-T-cell globulin in haematopoietic cell transplantation from matched unrelated donors: a randomised, open-label, multicentre phase 3 trial. Lancet Oncol 10:855–864. https://doi.org/10.1016/S1470-2045(09)70225-6
Grube M, Holler E, Weber D et al (2016) Risk factors and outcome of chronic graft-versus-host disease after allogeneic stem cell transplantation—results from a single-center observational study. Biol Blood Marrow Transplant 22:1781–1791. https://doi.org/10.1016/j.bbmt.2016.06.020
Jagasia MH, Greinix HT, Arora M et al (2015) National institutes of health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. The 2014 diagnosis and staging working group report. Biol Blood Marrow Transplant 21:389-401.e1. https://doi.org/10.1016/j.bbmt.2014.12.001
Kröger N, Solano C, Wolschke C et al (2016) Antilymphocyte globulin for prevention of chronic graft-versus-host disease. N Engl J Med 374:43–53. https://doi.org/10.1056/NEJMoa1506002
Lee SJ, Vogelsang G, Flowers MED (2003) Chronic graft-versus-host disease. Biol Blood Marrow Transplant 9:215–233. https://doi.org/10.1053/bbmt.2003.50026
Leisenring W, Friedman DL, Flowers MED et al (2006) Nonmelanoma skin and mucosal cancers after hematopoietic cell transplantation. J Clin Oncol 24:1119–1126. https://doi.org/10.1200/JCO.2005.02.7052
Martelin E, Volin L, Itälä-Remes M et al (2019) Incidence and risk factors of secondary cancers after allogeneic stem cell transplantation: analysis of a single centre cohort with a long follow-up. Bone Marrow Transplant 54:334–337. https://doi.org/10.1038/s41409-018-0290-6
Miller CS, Johnstone BM (2001) Human papillomavirus as a risk factor for oral squamous cell carcinoma: a meta-analysis, 1982–1997. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 91:622–635. https://doi.org/10.1067/moe.2001.115392
Novitzky-Basso I, Pasic I, Al-Shaibani Z et al (2020) Increased risk of secondary malignancy associated with the use of azathioprine for chronic graft-versus-host disease treatment. Blood 136:1–2. https://doi.org/10.1182/blood-2020-138927
Rizzo JD, Curtis RE, Socié G et al (2009) Solid cancers after allogeneic hematopoietic cell transplantation. Blood 113:1175–1183. https://doi.org/10.1182/BLOOD-2008-05-158782
Socié G, Rizzo JD (2012) Second solid tumors: screening and management guidelines in long-term survivors after allogeneic stem cell transplantation. Semin Hematol 49:4–9. https://doi.org/10.1053/j.seminhematol.2011.10.013
Sorror ML, Maris MB, Storb R et al (2005) Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 106:2912–2919. https://doi.org/10.1182/blood-2005-05-2004
Stewart BL, Storer B, Storek J et al (2004) Duration of immunosuppressive treatment for chronic graft-versus-host disease. Blood 104:3501–3506. https://doi.org/10.1182/blood-2004-01-0200
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
Material preparation, data collection and analysis were performed by Gruber Isabella. The duration of immunosuppressive therapy of chronic GVHD was analyzed by Appel Katharina. The manuscript was written by Gruber Isabella. Wolff Daniel provided patient samples and made substantial contributions to the conception, design and interpretation of the work. All authors contributed to the study conception and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
Gruber Isabella, Appel Katharina, Koelbl Oliver and Edinger Matthias have no financial or non-financial interests to disclose. Wolff Daniel received research support from Novartis and honoraria from Novartis, Sanofi, Incyte, Behring, Neovii, Takeda and Mallinckrodt.
Conflict of interest
Gruber Isabella, Appel Katharina, Koelbl Oliver and Edinger Matthias have no financial or non-financial interests to disclose. Wolff Daniel received research support from Novartis and honoraria from Novartis, Sanofi, Incyte, Behring, Neovii, Takeda and Mallinckrodt.
Ethical approval
The study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Regensburg (number, 20-1810-101, date, February 10, 2021).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Isabella, G., Katharina, A., Matthias, E. et al. Secondary solid malignancies and precancerous lesions after allogeneic hematopoietic stem cell transplantation using non-total body irradiation-based conditioning in acute myeloid leukemia. J Cancer Res Clin Oncol 150, 152 (2024). https://doi.org/10.1007/s00432-024-05679-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00432-024-05679-5