Abstract
Purpose
Pathogenic fusion events involving neurotrophic receptor tyrosine kinase (NTRK) have been described in ~ 2% of differentiated thyroid cancer (DTC). The selective tropomyosin receptor kinase (TRK) inhibitors entrectinib and larotrectinib have been approved in a tumor agnostic manner based on phase 1/2 clinical trials. In a real-world setting at five referral centers, we aimed to describe the prevalence of NTRK gene fusions and the efficacy and safety of TRK inhibitor treatment for non-medullary, advanced thyroid cancer (TC).
Methods
A total of 184 TC patients with testing for NTRK gene fusions were included. Progression-free survival (PFS) and overall survival (OS) probabilities were estimated using the Kaplan–Meier method in six patients with NTRK fusion-positive TC who underwent TRK inhibitor therapy.
Results
8/184 (4%) patients harbored NTRK gene fusions. Six patients with radioiodine (RAI)-refractory TC harboring NTRK1 (n = 4) and NTRK3 (n = 2) gene fusions were treated with larotrectinib. Five patients (83%) had received ≥ 1 prior systemic therapy and one patient did not receive prior systemic therapy. All patients had morphologically progressive disease before treatment initiation. Objective response rate was 83%, including two complete remissions. Median PFS from start of TRK inhibitor treatment was 23 months (95% confidence interval [CI], 0–57.4) and median OS was not reached (NR) (95% CI, NR). Adverse events were of grade 1–3.
Conclusion
The prevalence of NTRK gene fusions in our cohort of RAI-refractory TC is slightly higher than reported for all TC patients. Larotrectinib is an effective treatment option in the majority of NTRK gene fusion-positive advanced TC patients after prior systemic treatment and has a favorable safety profile.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with advanced, progressive thyroid cancer (TC) that is not amenable or refractory to radioiodine (RAI) therapy may require systemic treatment when palliative local therapeutic strategies or active surveillance are not an option (Haugen et al. 2016). The management of patients with locally advanced or metastatic non-medullary TC remains a therapeutic challenge. Recently, the therapeutic landscape of advanced TC has significantly changed with new targeted therapies becoming available.
Fusion events of neurotrophic receptor tyrosine kinase (NTRK) and rearranged during transfection (RET) drive tumorigenesis in certain cancers, including TC (Stransky et al. 2014; Koehler et al. 2020, 2022). Gene fusions of NTRK and RET result in an overexpression of chimeric tropomyosin receptor kinase (TRK) and RET proteins, respectively, with a constitutively active, ligand-independent downstream signaling and oncogenic potential. The reported presence of NTRK fusions in predominantly adult TC cohorts is low and ranges from 2.3 to 3.4% (Cancer Genome Atlas Research 2014; Lee et al. 2020; Liang et al. 2018; Solomon et al. 2020), with the highest prevalence in RAI-refractory differentiated thyroid cancer (DTC) (Ricarte-Filho et al. 2013). For patients in need of systemic treatment, the prevalence of NTRK fusions is not yet known.
The highly selective TRK inhibitors entrectinib and larotrectinib have been approved independent of the underlying tumor histology for patients with NTRK gene fusion-positive cancer in 2018 and 2019, respectively, based on phase 1/2 clinical trials (Doebele et al. 2020; Drilon et al. 2018; Hong et al. 2020). A pooled analysis of three phase 1/2 clinical trials with 159 adult and pediatric NTRK gene fusion-positive cancer patients treated with larotrectinib showed a remarkable objective response rate (ORR) of 79% including 24 patients (16%) with a complete response (CR) (Hong et al. 2020). In the subset of 28 patients with NTRK fusion-positive TC, the ORR was 75% including two patients (7%) with CR, and 29% in patients with anaplastic thyroid cancer (ATC) (Cabanillas et al. 2020).
In this retrospective study, we evaluated the prevalence of NTRK gene fusions in patients with non-medullary, advanced TC and described the patient characteristics, TRK inhibitor therapy practice, efficacy and treatment emergent adverse events (TEAE) in NTRK gene fusion-positive advanced TC patients receiving larotrectinib at three German and two Austrian tertiary care centers.
Methods: patients
Setting
Retrospectively collected data were obtained from records of patients diagnosed with non-medullary, advanced TC between 2002 and 2021 in three German and two Austrian tertiary care centers. Numbers for NTRK gene fusion testing were available from three German tertiary care centers. Data from two Austrian centers were not available. Data were collected as part of the German Study Group for Rare Tumors of the Thyroid gland approved by the ethics committee of the University of Würzburg [96/13] and subsequently by the ethics of all participating centers or by a waiver of consent, approved by the local institutional review boards for this minimal risk study. The data cut-off was 28 February 2023, and the median study follow-up was 19 months (range, 4–39).
Diagnostic strategy for NTRK fusions
The presence of NTRK fusions in patients receiving TRK inhibitor therapy was identified by commercially available oncology genomic profiling assays, including Archer Fusionplex Lung or OncologyResearch panels (IDT, ArcherDX), Oncomine Comprehensive Assay v3 (Thermo Fisher), Oncomine Focus Assay (Thermo Fisher), TruSight Tumor 170 kit (Illumina) and TruSight Oncology 500 kit (Illumina) from primary or metastatic tissue, according to the procedures established by each laboratory (Kirchner et al. 2020; Pfarr et al. 2020).
Data acquisition
Eligible patients were adults with advanced non-medullary TC receiving NTRK gene fusion event testing. Parameters reflecting TRK inhibitor therapy practice were collected in patients with molecular evidence of NTRK fusion-positive advanced TC who underwent TRK inhibitor therapy with a specific TRK inhibitor outside of a clinical trial. The selection of patients is shown in Fig. 1. Treatment and follow-up were done according to local clinical practice of participating centers. Response was assessed locally according to standard of care by 2-deoxy-2-[fluorine-18]fluoro-D-glucose integrated with CT (18F-FDG PET/CT), CT, magnetic resonance imaging (MRI) of the liver and bone scintigraphy and with serum thyroglobulin (Tg) testing every 3–6 months. Bone metastases were not considered as target lesions, except for new occurrence of bone metastases on treatment. Patients still alive were censored at last follow-up.
Statistical analysis
For categorical variables, we calculated relative and absolute frequencies; for numeric variables, we calculated medians and ranges. Progression-free survival (PFS) and overall survival (OS) probabilities were estimated using the Kaplan–Meier method. Microsoft Office Excel Version 16.55 was used for graphical presentation and additional analyses. Statistical analyses were performed with SPSS Version 26 (IBM, Chicago, IL, USA).
Results
NTRK gene fusion testing
In three centers with numerical data available, 184 patients with non-medullary, advanced TC were tested for the presence of NTRK gene fusions between 2018 and 2022. Overall, 8/184 (4%) were NTRK fusion positive, four patients required a systemic treatment and started TRK inhibitor treatment with larotrectinib. Two patients receiving TRK inhibitor treatment were included from two Austrian centers without data on the number of cases tested.
The analysis included patients with advanced TC with the following histological types based on the 2022 WHO classification of thyroid neoplasms (Baloch et al. 2022): 73 papillary thyroid cancer (PTC), 62 follicular thyroid cancer (FTC), 22 ATC, 21 poorly differentiated thyroid cancer (PDTC), and 6 oncocytic TC. Supplementary Table 1 shows NTRK gene fusion partners of all patients with NTRK gene fusions.
Characteristics of patients with TRK inhibitor treatment
Baseline clinical characteristics of the six treated patients (5 females, 1 male), all of whom received larotrectinib, are shown in Table 1. Median follow-up from initial TC diagnosis was 7 years (range, 1–18) and median follow-up from start of larotrectinib therapy 19 months (range, 4–39). NTRK fusions discovered involved TRKA (NTRK1) in 4 patients (67%), and TRKC (NTRK3) in 2 patients (33%) with 5 unique fusion partners in primary (3/6, 50%) or metastatic tumor tissue (3/6, 50%).
Tumor-specific therapy
Five patients (83%) underwent total thyroidectomy and RAI therapy as initial therapy. One patient (17%) received hemithyroidectomy. The patient with ATC (17%) did not receive RAI therapy. All patients had RAI-refractory disease by the time of study inclusion. Before larotrectinib therapy, 4 patients (67%) received multi-tyrosine kinase inhibitor (MKI) treatment with sorafenib and/or lenvatinib. Two patients (50%) received one MKI (lenvatinib) and 2 patients (50%) received two MKIs (sorafenib followed by lenvatinib) before larotrectinib treatment. One patient (17%) received selitrectinib (LOXO-195) on a “named-patient basis”, a next-generation pan-TRK inhibitor after treatment with larotrectinib. Treatment for local recurrence before larotrectinib initiation was surgery in 2 patients, and external beam radiation of the neck in 4 patients. Treatment of distant metastases included surgery in 5, and external beam radiation in 2 patients. One patient did not receive systemic treatment, treatment for local recurrence or treatment of distant metastases before study inclusion.
Treatment characteristics and response to larotrectinib treatment are summarized in Table 2. Median age at larotrectinib initiation was 69 years (range, 49–74) and median time between initial diagnosis and treatment initiation was 49 months (range, 12–209). Indication for TRK inhibitor treatment was progressive disease (PD) during MKI treatment in 4 patients, PD during treatment with another TRK inhibitor in 1 patient and PD in metastatic RAI-refractory DTC without prior systemic treatment in 1 patient. At the time of larotrectinib initiation, 4 patients had local regional lymph node metastases, and 6 distant metastases (brain 1 [17%], mediastinal lymph nodes 2 [33%], lung 5 [83%], liver 2 [33%], pleura 2 [33%], and osteolytic bone metastases 3 [50%]). All patients with bone metastases received anti-resorptive therapy (ART). Two (67%) patients received bisphosphonates, and 1 (33%) patient was sequentially treated with bisphosphonates and denosumab.
Efficacy of larotrectinib treatment
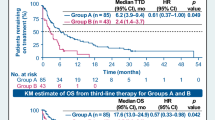
The median PFS from start of TRK inhibitor treatment was 23 months (95% confidence interval [95% CI], 0–57.4; Fig. 2a), and median OS was not reached (NR) (95% CI, NR; Fig. 2b). Metastatic sites with the best morphological response were bone metastases (2 [67%]; n = 3), lymph nodes (2 [67%]; n = 3), and pulmonal metastases (2 [67%]; n = 3). At the data cut-off, 2 patients (33%) were still receiving TRK inhibitor treatment, 3 patients (50%) discontinued due to PD, and 1 patient (17%) discontinued due to TEAE grade 3 (gait instability with pre-existing peripheral polyneuropathy). One patient (17%) had died, and 2 patients (33%) were lost to follow-up.
Safety and tolerability of larotrectinib
Four (67%) patients showed TEAE, of all the adverse events being of grades 1–3. One patient discontinued treatment due to TEAEs. The most frequently reported drug-related TEAE were weight gain (33%), and fatigue (33%). Further TEAEs included anemia (17%), dizziness (17%), elevated liver enzymes (17%), gait instability (17%), and polyneuropathy (17%).
Selitrectinib treatment
One patient received the investigational TRK inhibitor selitrectinib outside of a clinical trial after treatment with larotrectinib due to PD. After 3 months of treatment and continuation of PD, the patient was switched to best supportive care. TEAE included nausea and fatigue grade 3.
Case description
A 67-year-old female patient diagnosed initially with FTC in 11/2017 presented for follow-up of advanced FTC with metastatic sites including lymph nodes, liver and lung. Time between initial diagnosis and evidence of RAI-refractory disease was 5 months. Follow-up with 18F-FDG PET/CT revealed PD and an increasing serum Tg level of 5231 ng/mL (reference range, 3.5–77 ng/mL) compared to 500 ng/mL one month before. Due to PD with detection of new bone and pituitary metastases with secondary adrenal insufficiency, hydrocortisone replacement and tumor-specific treatment with lenvatinib 24 mg/d was initiated. At that time, the formalin-fixed paraffin-embedded tumor sample underwent testing with the Oncomine Comprehensive Assay v3. The analysis revealed a SQSTM1/NTRK3 gene fusion and reference pathology led to the revised diagnosis of oncocytic TC with poorly differentiated features. After 7 months of lenvatinib, treatment was switched to larotrectinib 100 mg twice daily due to PD and increasing Tg levels of 11,160 ng/mL. First follow-up revealed partial remission (PR). Biochemical and radiologic response are summarized in Fig. 3.
Biochemical and radiologic responses to larotrectinib a Tumor progression with Tg increased to 5231 ng/mL (reference range, 3.5–77 ng/mL) despite lenvatinib treatment. Further molecularly informed treatment with the TRK inhibitor larotrectinib at a dose of 100 mg BID decreased Tg to 1690 ng/mL within four weeks. The further increase of serum Tg after start of NTRK inhibitor treatment was interpreted as a functional sign of redifferentiation. As of the time of this report, at 30 months from the start of larotrectinib, the patient continues on treatment with SD and no TEAE b Brain MRI reveals a new pituitary metastasis during lenvatinib treatment c A MRI of the liver during lenvatinib treatment reveals disseminated hepatic metastatic disease d Significant tumor shrinkage in the course of therapy with larotrectinib e Radiologic follow-up revealed PR of liver metastases. BID bis in die
Discussion
Here, we studied the prevalence of NTRK gene fusion events in TC patients requiring systemic therapy and report real-world clinical data from patients with NTRK gene fusion-positive non-medullary, advanced TC who received treatment with larotrectinib outside of a clinical trial.
NTRK fusions in TC are rare with a reported frequency of about 2% (Cancer Genome Atlas Research 2014; Rosen et al. 2020). The slightly higher frequency of 4% in our study cohort is most likely due to selection bias. Nevertheless, the actual frequency of NTRK fusions in TC could be underestimated due to limitations of currently available testing modalities (Solomon et al. 2020). Therefore, a recent multicentric study tested the comparative performance of different methodologies regarding their ability to detect NTRK gene fusions and suggested a test algorithm based on entity (Pfarr et al. 2020). Moreover, in recent years, multinational ring trials for NTRK fusion detection were available to test the suitability of the, respectively, used method in the participating laboratories (Kirchner et al. 2020).
The female predominance of 83% in our treated cohort is consistent with the data from Chu et al., who characterized 11 NTRK-rearranged TC by clinicopathologic and molecular features and observed a female predominance of 73% (Chu et al. 2020). Furthermore, they observed NTRK-rearrangement only in tumors which had been originally diagnosed as PTC (Chu et al. 2020). In comparison, the series by Cabanillas et al., Fazeli et al., and Park et al. comprises patients with ATC/FTC, ATC/PDTC, and PTC/PDTC/ATC, respectively (Cabanillas et al. 2020; Fazeli et al. 2020; Park et al. 2022). The proportion of different histologic subtypes in our complete NTRK fusion-positive cohort of 10 patients is in accordance with the published data, confirming that NTRK fusions occur predominantly in PTC, but with a lesser frequency also in less differentiated tumors.
Among the treated patients, the frequency of distant metastases at initial diagnosis (40%) is comparable to the data from Chu et al. and Fazeli et al., reporting distant metastases in about 25% and 38%, respectively (Chu et al. 2020; Fazeli et al. 2020). The rate of NTRK1 fusions in our cohort of 10 patients is higher at 60% compared to the recently published series (Cabanillas et al. 2020; Chu et al. 2020; Fazeli et al. 2020; Park et al. 2022). Though, all PDTC and ATC patients showed a NTRK3 fusion which is in accordance with reported results by Fazeli et al. showing the NTRK3 fusion to be the most common NTRK fusion in ATC and PDTC patients (7/8, 87%) (Fazeli et al. 2020). No case showed a NTRK2 fusion. This is consistent with published data from non-medullary TC patients (Cabanillas et al. 2020; Chu et al. 2020; Fazeli et al. 2020), except for the recently published case series, which showed a NTRK2 fusion in 1/4 of the patients (Park et al. 2022).
The efficacy of larotrectinib in our retrospective analysis is in accordance with previously reported results; the ORR was 83% versus 75% across various TRK fusion-positive tumor types in a primary analysis set of 55 patients in a phase 1/2 basket trial and 79% in a pooled analysis of three phase 1/2 clinical trials (Drilon et al. 2018; Hong et al. 2020). Data from patients with locally advanced or metastatic NTRK fusion-positive TC pooled from two larotrectinib clinical trials published in abstract form showed an ORR of 75% including patients with ATC, and 90% only in patients with DTC (Cabanillas et al. 2020). We found CR in 33%, and PR in 50%, which translated into a PFS of 23 months. Our case report of a patient with oncocytic TC and evidence of poorly differentiated features confirms that druggable fusion events may be present in oncocytic TC with similar responsiveness to TRK inhibitor therapy.
No patient showed primary resistance to larotrectinib, defined as a best response of PD (Drilon et al. 2018). Acquired resistance to larotrectinib, defined as PD after objective response or SD for at least 6 months (Drilon et al. 2018; Jackman et al. 2010), was observed in 1 patient (16%). The patient underwent repeated testing to determine the mechanisms of acquired resistance to larotrectinib. Histopathology of a soft tissue sample of the neck revealed avital squamous keratinized epithelium with evidence of squamous cell carcinoma. A second next generation sequencing (NGS) performed did not exhibit the same mutation profile as the initial tissue sample of a lung metastasis: the presence of a NTRK1 fusion was not confirmed, potentially explaining the lack of response in this patient. Whether the histopathological result represents squamous cell differentiation at metastatic site before or during treatment with larotrectinib or involvement by concomitant squamous cell carcinoma from another organ remains a matter of debate. Furthermore, no known larotrectinib-resistant mutation was detected. Drilon et al. found acquired resistance to larotrectinib in 2 patients: repeated testing after PD revealed mutations in the kinase domain affecting the NTRK gene involved in the fusion (Drilon et al. 2017). A next-generation TRK inhibitor can potentially overcome acquired resistance to earlier-generation TRK inhibitors (Drilon et al. 2017).
Based on recent evidence long-term administration of larotrectinib appears feasible (Hong et al. 2020). Maximum treatment duration in our cohort was 30 months and two patients are still receiving treatment with larotrectinib at data cut-off. The favorable safety and tolerability profile in our cohort is consistent with the published data without grade 4/5 adverse events in our cohort (Drilon et al. 2018).
Our study of six NTRK fusion-positive advanced TC patients treated with larotrectinib at five specialized centers in Germany and Austria has some limitations: missing data due to its retrospective nature, small patient number, lack of systematic follow-up, heterogeneity of patient management, and the evaluation of imagings by different radiologists.
Conclusion
We demonstrate that larotrectinib, a highly selective TRK inhibitor, is an effective drug in patients with locally advanced or metastatic NTRK fusion-positive TC, regardless of the therapy line. Additional real-world data from a larger cohort of TC patients with a longer follow-up are needed, as well as data comparing larotrectinib to standard of care.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baloch ZW, Asa SL, Barletta JA, Ghossein RA, Juhlin CC, Jung CK, Mete O (2022) Overview of the 2022 WHO classification of thyroid neoplasms. Endocr Pathol 33(1):27–63. https://doi.org/10.1007/s12022-022-09707-3
Cabanillas ME, Drilon A, Farago AF, Brose MS, McDermott R, Sohal D, Waguespack SG (2020) 1916P Larotrectinib treatment of advanced TRK fusion thyroid cancer. Ann Oncol 31:S1086
Cancer Genome Atlas Research Network (2014) Integrated genomic characterization of papillary thyroid carcinoma. Cell 159(3):676–690. https://doi.org/10.1016/j.cell.2014.09.050
Chu YH, Dias-Santagata D, Farahani AA, Boyraz B, Faquin WC, Nose V, Sadow PM (2020) Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Mod Pathol 33(11):2186–2197. https://doi.org/10.1038/s41379-020-0574-4
Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, trial, investigators (2020) Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet Oncol 21(2):271–282. https://doi.org/10.1016/S1470-2045(19)30691-6
Drilon A, Nagasubramanian R, Blake JF, Ku N, Tuch BB, Ebata K, Hyman DM (2017) A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov 7(9):963–972. https://doi.org/10.1158/2159-8290.CD-17-0507
Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Hyman DM (2018) Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 378(8):731–739. https://doi.org/10.1056/NEJMoa1714448
Fazeli S, Dadu R, Waguespack SG, Sherman SI, Busaidy NL, Hu MI, Cabanillas M (2020) MON-491 TRK-fusion thyroid cancer: a clinical overview in a large population at a single cancer center. J Endocr Soc. https://doi.org/10.1210/jendso/bvaa046.328
Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Wartofsky L (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133. https://doi.org/10.1089/thy.2015.0020
Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, Drilon A (2020) Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol 21(4):531–540. https://doi.org/10.1016/S1470-2045(19)30856-3
Jackman D, Pao W, Riely GJ, Engelman JA, Kris MG, Janne PA, Miller VA (2010) Clinical definition of acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in non-small-cell lung cancer. J Clin Oncol 28(2):357–360. https://doi.org/10.1200/JCO.2009.24.7049
Kirchner M, Glade J, Lehmann U, Merkelbach-Bruse S, Hummel M, Lehmann A, Pfarr N (2020) NTRK testing: first results of the QuiP-EQA scheme and a comprehensive map of NTRK fusion variants and their diagnostic coverage by targeted RNA-based NGS assays. Genes Chromosom Cancer 59(8):445–453. https://doi.org/10.1002/gcc.22853
Koehler VF, Adam P, Frank-Raue K, Raue F, Berg E, Hoster E, Spitzweg C (2020) Real-world efficacy and safety of Cabozantinib and Vandetanib in advanced medullary thyroid cancer. Thyroid. https://doi.org/10.1089/thy.2020.0206
Koehler VF, Adam P, Fuss CT, Jiang L, Berg E, Frank-Raue K, Parathyroid G (2022) Treatment of RET-positive advanced medullary thyroid cancer with multi-tyrosine kinase inhibitors-a retrospective multi-center registry analysis. Cancers (basel) 14(14):3405. https://doi.org/10.3390/cancers14143405
Lee YC, Chen JY, Huang CJ, Chen HS, Yang AH, Hang JF (2020) Detection of NTRK1/3 rearrangements in papillary thyroid carcinoma using immunohistochemistry, fluorescent in situ hybridization, and next-generation sequencing. Endocr Pathol 31(4):348–358. https://doi.org/10.1007/s12022-020-09648-9
Liang J, Cai W, Feng D, Teng H, Mao F, Jiang Y, Sun ZS (2018) Genetic landscape of papillary thyroid carcinoma in the Chinese population. J Pathol 244(2):215–226. https://doi.org/10.1002/path.5005
Park JC, Ashok A, Liu C, Kang H (2022) Real-World Experience of NTRK fusion-positive thyroid cancer. JCO Precis Oncol 6:e2100442. https://doi.org/10.1200/PO.21.00442
Pfarr N, Kirchner M, Lehmann U, Leichsenring J, Merkelbach-Bruse S, Glade J, Stenzinger A (2020) Testing NTRK testing: wet-lab and in silico comparison of RNA-based targeted sequencing assays. Genes Chromosomes Cancer 59(3):178–188. https://doi.org/10.1002/gcc.22819
Ricarte-Filho JC, Li S, Garcia-Rendueles ME, Montero-Conde C, Voza F, Knauf JA, Fagin JA (2013) Identification of kinase fusion oncogenes in post-Chernobyl radiation-induced thyroid cancers. J Clin Invest 123(11):4935–4944. https://doi.org/10.1172/JCI69766
Rosen EY, Goldman DA, Hechtman JF, Benayed R, Schram AM, Cocco E, Hyman DM (2020) TRK fusions are enriched in cancers with uncommon histologies and the absence of canonical driver mutations. Clin Cancer Res 26(7):1624–1632. https://doi.org/10.1158/1078-0432.CCR-19-3165
Solomon JP, Linkov I, Rosado A, Mullaney K, Rosen EY, Frosina D, Hechtman JF (2020) NTRK fusion detection across multiple assays and 33,997 cases: diagnostic implications and pitfalls. Mod Pathol 33(1):38–46. https://doi.org/10.1038/s41379-019-0324-7
Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C (2014) The landscape of kinase fusions in cancer. Nat Commun 5:4846. https://doi.org/10.1038/ncomms5846
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Munich Clinician Scientist Program Track FöFoLe + , Reg.-Nr. 044 of the medical faculty of the LMU Munich and unrestricted grants from Lilly, Eisai, and Bayer in support of the registry database.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by VFK, JA, NS and LK. The first draft of the manuscript was written by VFK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
Viktoria F. Koehler has received honoraria for lectures and travel expenses from Novartis and Sanofi. Christine Koch received honoraria for advisory boards and lectures from Ipsen, Eisei, MSD, BMS, Astra Zeneca, Bayer. Philipp Ivanyi has received advisory fees from BMS, Bayer, ClinSol, Deciphera, EISAI, EMD-Serono, EUSA, H5-Oncology, Ipsen, Merck Serono (Global), Metaplan, MSD, Onkowissen, Pfizer, Roche, honoraria for lectures from AIM, Apogepha, Astra Zeneca, Astella, BMS, Bayer (+ Europe, Global), CORE2ED, Deciphera, DKG-Onkoweb, EISAI, EUSA, FoFM, Id-Institut, Ipsen (Europe), Merck Serono (+ Europe, Global), MSD, MedKom, MTE-Academy, MedWiss, New Concept Oncology, Onkowissen-tv.de, Pharma Mare, Pfizer, Roche, ThinkWired!, Schmitz-Communikation, StreamedUP!, Solution Academy, Vivantis, research grants from AIO, AstraZeneca, BMS, GSK, Ipsen, Lilly, Merck Serono, Niedersächsische Krebsgesellschaft, Novartis, EUSA, EISAI, Pfizer, MSD, Roche, Stiftung Immunonkologie, Wilhelm Sander Stiftung, travel grants from BB-Biotech, BMS, Bayer, Deutsche Gesellschaft für Thoraxchirurgie, EUSA, Ipsen, Merck, Pharma Mare, non-financial grants from Member of Germen Working Party Medical Oncology (AIO), Member of the German Cancer Society, ASCO Member, ESMO Member, Member of Oncological Working Party Hannover (OAK), Spokesman Interdisciplinary Working Party – Kidney Cancer (IAGN-DKG), Steering Board Immunooncology Cooperative Group (ICOG-H), Clinical Trail Streering Committee CCC-H. Lukas Käsmann has received honoraria for lectures from AMGEN and the German Cancer Society, and an unrestricted research grant from AstraZeneca. Melanie-Christin Demes received honoraria from talks and advisory board role from Amgen, AstraZeneca, Bayer, Biocartis, Diaceutics, Roche, Sophia Genetics und ThermoFisher. Christine Spitzweg has received honoraria for advisory boards and lectures from Ipsen, Lilly, Bayer, Eisai, Genzyme. Matthias Kroiss has received institutional research support from Ipsen, Loxo Oncology and Lilly, travel support from Eisai, Ipsen, HRA Pharma, MCI Germany and Lilly, honoraria for lectures from Bristol-Myers Squibb, Eisai, Lilly, and MSD and consultancy honoraria from Lilly and Bayer. Josefine Achterfeld, Natalie Sandner, Jonas Paul Wiegmann, Renate Pusch, Dominik Wolf, Mihaela Chirica, Thomas Knösel, Joerg Kumbrink, Thomas J Vogl, Gesine Meyer, and Joerg Bojunga have no competing financial interests.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of University Würzburg [96/13] and subsequently by the ethics of all participating centers or by a waiver of informed consent, approved by the local institutional review boards for this minimal risk study.
Consent to participate
A waiver of informed consent was applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Koehler, V.F., Achterfeld, J., Sandner, N. et al. NTRK fusion events and targeted treatment of advanced radioiodine refractory thyroid cancer. J Cancer Res Clin Oncol 149, 14035–14043 (2023). https://doi.org/10.1007/s00432-023-05134-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-023-05134-x