Abstract
Purpose
The purpose of this study is to compare response rates of lenvatinib and atezolizumab plus bevacizumab, in first-line real-world setting.
Methods
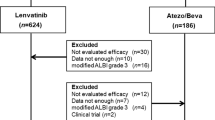
Overall cohort included Western and Eastern hepatocellular carcinoma (HCC) patient populations from 46 centres in 4 countries (Italy, Germany, Japan, and Republic of Korea).
Results
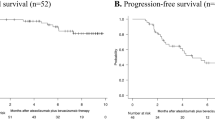
1312 patients were treated with lenvatinib, and 823 patients were treated with atezolizumab plus bevacizumab. Objective response rate (ORR) was 38.6% for patients receiving lenvatinib, and 27.3% for patients receiving atezolizumab plus bevacizumab (p < 0.01; odds ratio 0.60). For patients who achieved complete response (CR), overall survival (OS) was not reached in both arms, but the result from univariate Cox regression model showed 62% reduction of death risk for patients treated with atezolizumab plus bevacizumab (p = 0.05). In all multivariate analyses, treatment arm was not found to be an independent factor conditioning OS. Comparing ORR achieved in the two arms, there was a statistically significant difference in favor of lenvatinib compared to atezolizumab plus bevacizumab in all subgroups except for Eastern patients, Child–Pugh B patients, presence of portal vein thrombosis, α-feto-protein ≥ 400 ng/mL, presence of extrahepatic disease, albumin–bilirubin (ALBI) grade 2, and no previous locoregional procedures.
Conclusion
Lenvatinib achieves higher ORR in all patient subgroups. Patients who achieve CR with atezolizumab plus bevacizumab can achieve OS so far never recorded in HCC patients. This study did not highlight any factors that could identify patient subgroups capable of obtaining CR.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- HCC:
-
Hepatocellular carcinoma
- ORR:
-
Objective response rate
- CR:
-
Complete response
- OS:
-
Overall survival
- HR:
-
Hazard ratio
- CI:
-
Confidence interval
- PFS:
-
Progression-free survival
- BCLC:
-
Barcelona clinic liver cancer
- PR:
-
Partial response
- FU:
-
Follow-up
- CP:
-
Child–Pugh
- TACE:
-
Trans-arterial chemoembolization
- ECOG:
-
Eastern Cooperative Oncology Group
- SD:
-
Stable disease
- PD:
-
Progressed disease
- ALBI:
-
Albumin–bilirubin
- NLR:
-
Neutrophil–lymphocyte ratio
- αFP:
-
Alpha-feto-protein
- PVT:
-
Portal vein thrombosis
- EHD:
-
Extrahepatic disease
References
Burgio V, Iavarone M, Di Costanzo GG, Marra F, Lonardi S, Tamburini E et al (2021) Real-life clinical data of lenvatinib versus sorafenib for unresectable hepatocellular carcinoma in Italy. Cancer Manag Res 13:9379–9389
Casadei-Gardini A, Scartozzi M, Tada T, Yoo C, Shimose S, Masi G et al (2021) Lenvatinib versus sorafenib in first-line treatment of unresectable hepatocellular carcinoma: an inverse probability of treatment weighting analysis. Liver Int 00:1–9
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS et al (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10(1):25–34
Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY et al (2022) Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol 76(4):862–873
Ducreux M, Zhu AX, Cheng A-L et al (2021) IMbrave150: Exploratory analysis to examine the association between treatment response and overall survival (OS) in patients (pts) with unresectable hepatocellular carcinoma (HCC) treated with atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor). J Clin Oncol 39(15):4071
Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim T-Y et al (2020) Complete responses (CR) in patients receiving atezolizumab (atezo) + bevacizumab (bev) versus sorafenib (sor) in IMbrave150: a phase III clinical trial for unresectable hepatocellular carcinoma (HCC). J Clin Oncol 38(15):4596
Freites-Martinez A, Santana N, Arias-Santiago S, Viera A (2021) Using the common terminology criteria for adverse events (CTCAE - Version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermosifiliogr (Engl Ed) 112(1):90–92.
Han Y, Zhi WH, Xu F, Zhang CB, Huang XQ, Luo JF (2021) Selection of first-line systemic therapies for advanced hepatocellular carcinoma: a network meta-analysis of randomized controlled trials. World J Gastroenterol 27(19):2415–2433
Hatanaka T, Kakizaki S, Nagashima T, Namikawa M, Tojima H, Shimada Y et al (2020) Analyses of objective response rate, progression-free survival, and adverse events in hepatocellular carcinoma patients treated with lenvatinib: a multicenter retrospective study. Hepatol Res 50:382–395
Hiraoka A, Kumada T, Atsukawa M, Hirooka M, Tsuji K, Ishikawa T et al (2019) Prognostic factor of lenvatinib for unresectable hepatocellular carcinoma in real-world conditions-multicenter analysis. Cancer Med 8:3719–3728
Iwamoto H, Shimose S, Noda Y, Shirono T, Niizeki T, Nakano M et al (2021) Initial experience of atezolizumab plus bevacizumab for unresectable hepatocellular carcinoma in real-world clinical practice. Cancers 13:2786
Keenan BP, Fong L, Kelley RK (2019) Immunotherapy in hepatocellular carcinoma: the complex interface between inflammation, fibrosis, and the immune response. J Immunother Cancer 7:1–13
Khoja L, Kibiro M, Metser U, Gedye C, Hogg D, Butler MO et al (2016) Patterns of response to anti-PD-1 treatment: an exploratory comparison of four radiological response criteria and associations with overall survival in metastatic melanoma patients. Br J Cancer 15:1186–1192
Kim BK, Cheon J, Kim H, Kang B, Ha Y, Kim DY et al (2022) Atezolizumab/bevacizumab vs. lenvatinib as first-line therapy for unresectable hepatocellular carcinoma: a real-world, multi-center study. Cancers 14:1747
Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F et al (2018) Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 391(10126):1163–1173
Kudo M, Finn RS, Qin S, Han K-H, Ikeda K, Cheng A-L et al (2019a) Analysis of survival and objective response (OR) in patients with hepatocellular carcinoma in a phase III study of lenvatinib (REFLECT). J Clin Oncol 37(4):186
Kudo M, Ueshima K, Chan S, Minami T, Chishina H, Aoki T et al (2019b) Lenvatinib as an initial treatment in patients with intermediate-stage hepatocellular carcinoma beyond up-to-seven criteria and child–pugh A liver function: a proof-of-concept study. Cancers (basel) 11(8):1084
Liu JKH, Irvine AF, Jones RL, Samson A (2022) Immunotherapies for Hepatocellular Carcinoma. Cancer Med 11:571–591
Llovet JM, Bru C, Bruix J (1999) Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis 19:329–338
Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF et al (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359(4):378–390
Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S et al (2021) Hepatocellular Carcinoma. Nat Rev Dis Primers 7(1):6
Maruta S, Ogasawara S, Ooka Y, Obu M, Inoue M, Itokawa N et al (2020) Potential of lenvatinib for an expanded indication from the REFLECT trial in patients with advanced hepatocellular carcinoma. Liver Cancer 9:382–396
Ogushi K, Chuma M, Uojima H, Hidaka H, Numata K, Kobayashi S et al (2020) Safety and efficacy of lenvatinib treatment in Child-Pugh A and B patients with unresectable hepatocellular carcinoma in clinical practice: a multicenter analysis. Clin Exp Gastroenterol 13:385–396
Ohki T, Sato K, Kondo M, Goto E, Sato T, Kondo Y et al (2020) Impact of adverse events on the progression-free survival of patients with advanced hepatocellular carcinoma treated with lenvatinib: a multicenter retrospective study. Drugs Real World Outcomes 7:141–149
Prieto J, Melero I, Sangro B (2015) Immunological landscape and immunotherapy of hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol 12:681–700
Rapposelli IG, De Matteis S, Lanuti P, Valgiusti M, Bartolini G, Ulivi P et al (2021a) Heterogeneity of response and immune system activity during treatment with nivolumab in hepatocellular carcinoma: results from a single-institution retrospective analysis. Cancers 13:213
Rapposelli IG, Tada T, Shimose S, Burgio V, Kumada T, Iwamoto H et al (2021b) Adverse events as potential predictive factors of activity in patients with advanced hepatocellular carcinoma treated with lenvatinib. Liver Int 41(12):2997–3008
Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á et al (2022) BCLC strategy for prognosis prediction and treatment recommendation: the 2022 update. J Hepatol 76(3):681–693
Rimini M, Shimose S, Lonardi S, Tada T, Masi G, Iwamoto H et al (2021) Lenvatinib versus Sorafenib as first-line treatment in hepatocellular carcinoma: a multi-institutional matched case-control study. Hepatol Res 51(12):1229–1241
Rimini M, Liscia N, Burgio V, Casadei-Gardini A (2022) Why does survival of hepatocellular carcinoma patients remain so low? Key stumbling blocks and questions in preclinical and clinical development. Expert Opin Investig Drugs 31(5):483–494
Sasaki R, Fukushima M, Haraguchi M, Miuma S, Miyaaki H, Hidaka M et al (2019) Response to lenvatinib is associated with optimal relative dose intensity in hepatocellular carcinoma: experience in clinical settings. Cancers 11:1769
Shimose S, Kawaguchi T, Tanaka M, Iwamoto H, Miyazaki K, Moriyama E et al (2020) Lenvatinib prolongs the progression-free survival time of patients with intermediate-stage hepatocellular carcinoma refractory to transarterial chemoembolization: a multicenter cohort study using data mining analysis. Oncol Lett 20(3):2257–2265
Shimose S, Iwamoto H, Tanaka M, Niizeki T, Shirono T, Noda Y et al (2021) Alternating lenvatinib and trans-arterial therapy prolongs overall survival in patients with intermediate stage hepatocellular carcinoma: a propensity score matching study. Cancers 13:160
Sho T, Suda G, Ogawa K, Shigesawa T, Suzuki K, Nakamura A et al (2020) Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol Res 50:966–977
Sonbol MB, Riaz IB, Naqvi SAA (2020) Systemic therapy and sequencing options in advanced hepatocellular carcinoma: a systematic review and network meta-analysis. JAMA Oncol 6(12):e204930
Spallanzani A, Orsi G, Andrikou K, Gelsomino F, Rimini M, Riggi L et al (2018) Lenvatinib as a therapy for unresectable hepatocellular carcinoma. Expert Rev Anticancer Ther 18(11):1069–1076
Tazdait M, Mezquita L, Lahmar J, Ferrara R, Bidault F, Ammari S et al (2018) Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 88:38–47
Ueshima K, Nishida N, Hagiwara S, Aoki T, Minami T, Chishina H et al (2019) Impact of baseline ALBI grade on the outcomes of hepatocellular carcinoma patients treated with lenvatinib: a multicenter study. Cancers 11:952
Wherry EJ (2011) T cell exhaustion. Nat Immunol 12:492–499
Zambrana F, Carril-Ajuria L, Gómez de Liaño A, Martinez Chanza N, Manneh R, Castellano D et al (2021) Complete response and renal cell carcinoma in the immunotherapy era: the paradox of good news. Cancer Treat Rev 99:102239
Acknowledgements
Nothing to declare.
Funding
The authors declare that no funds, grants, or other support was received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AC-G, MP, and MR contributed to the study conception and design. Material preparation and data collection were performed by all authors. Analysis and interpretation of data were performed by AC-G, MP, and MR. The first draft of the manuscript was written by AC-G, MP, and MR and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
M.K. has received grants from Taiho Pharmaceuticals, Chugai Pharmaceuticals, Otsuka, Takeda, Sumitomo Dainippon-Sumitomo, Daiichi Sankyo, AbbVie, Astellas Pharma, and Bristol-Myers Squibb; has received grants and personal fees from MSD, Eisai, and Bayer, and is an adviser for MSD, Eisai, Bayer, Bristol-Myers Squibb, Eli Lilly and ONO Pharmaceutical. L.R. has received consulting fees from Amgen, ArQule, AstraZeneca, Basilea, Bayer, BMS, Celgene, Eisai, Exelixis, Genenta, Hengrui, Incyte, Ipsen, IQVIA, Lilly, MSD, Nerviano Medical Sciences, Roche, Sanofi, Servier, Taiho Oncology, Zymeworks; lecture fees from AbbVie, Amgen, Bayer, Eisai, Gilead, Incyte, Ipsen, Lilly, Merck Serono, Roche, Sanofi; travel expenses from AstraZeneca; and institutional research funding from Agios, ARMO BioSciences, AstraZeneca, BeiGene, Eisai, Exelixis, Fibrogen, Incyte, Ipsen, Lilly, MSD, Nerviano Medical Sciences, Roche, Zymeworks. F.P. has received consulting or lecture fees from Consulting or lecture fees in the last two years from: Astrazeneca, Bayer, Bracco, EISAI, ESAOTE, Exact Sciences, IPSEN; MSD; Roche, Samsung, Tiziana Life Sciences. M.S. is an advisor for MSD, Eisai, MERCK, SERVIER, and AMGEN. A.C-G. has received grants and personal fees from MSD, Eisai, Bayer, and is an advisor for MSD, Eisai, Bayer, Bristol-Myers Squibb, AstraZeneca and GSK. The other authors have no relevant financial or non-financial interests to disclose.
Ethics statement
Study was approved by Ethics Committee at each center (number of approval code 113/INT/2021 by the IRCCS San Raffaele Hospital Ethic Committee), complied with the provisions of the Good Clinical Practice guidelines and the Declaration of Helsinki and local laws, and fulfilled the Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Persano, M., Rimini, M., Tada, T. et al. Clinical outcomes with atezolizumab plus bevacizumab or lenvatinib in patients with hepatocellular carcinoma: a multicenter real-world study. J Cancer Res Clin Oncol 149, 5591–5602 (2023). https://doi.org/10.1007/s00432-022-04512-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04512-1