Abstract
Objective
To explore the characteristics of cancer patients who cryopreserved sperm/testicular tissue samples in the Cryobank of Charité-Universitätsmedizin Berlin between 2004 and 2019, and the ART utilization rate with associated outcomes.
Methods
Retrospective data were available for 506 cancer patients, of which 46 (9.1%) had used their samples for artificial reproductive technologies (ART). Corresponding cycle information was collected from external fertility centers.
Results
Our cohort included 53/506 (10.5%) patients aged < 18 years at diagnosis. While adolescents and adults mainly banked sperm, adolescents showed higher rates of testicular tissue cryopreservation before (11.8%, 6/51 vs. 6.4%, 26/406) and after treatment (16.7%, 4/24 vs. 7.8%, 13/167). At study conduction, storage had been ended for 44.8% (269/601) of samples. The majority of samples used for ART were requested within the first 3 years after cryopreservation (71.5%, 28/39, range = 0–12 years). Pregnancy rate was 51.4% (19/37 cycles), resulting in 11 singleton births, 3 twin pairs, and 4 miscarriages.
Conclusion
With the new advantage of public health insurance coverage of fertility preservation (FP) in Germany, an increased utilization has already been noticed in our center, emphasizing the necessity of further knowledge for individual counseling. Adolescent cancer patients need to be addressed specifically, as these patients show especially low cryopreservation rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A cancer diagnosis and its necessary treatment can impair spermatogenesis in male (long-term) survivors or even lead to irreversible infertility (Okada and Fujisawa 2018). Cryopreservation of sperm or testicular tissue are well-established techniques of fertility preservation (FP) in cancer patients and current guidelines recommend its use before initiation of a potentially gonadotoxic treatment (Dittrich et al. 2018; Lambertini et al. 2020). Despite these recommendations, the rate of FP utilization in male cancer patients is low (Balcerek et al. 2020; Chong et al. 2010). Treating physicians may experience a variety of barriers related to counseling of their patients on the risk of fertility impairment and on FP options, contributing to low FP utilization rates in patients (Halpern et al. 2020). The majority of cancer patients, including adolescents (Korte et al. 2020), however, desire to have (future) biological children (Schover et al. 2002). The inability to achieve a pregnancy may lead to reduced psychosocial well-being (Maroufizadeh et al. 2018). While natural conception following a cancer diagnosis and oncologic treatment is possible, difficulties in achieving a pregnancy remain common and survivors may require support from assisted reproductive technologies (ART). Live-birth rates following ART in cancer patients are—regardless of whether fresh or cryopreserved sperm cells are used—comparable to rates in infertile couples in Europe (Papler et al. 2021). Adequate patient counseling, early FP, and surveillance of fertility following cancer treatment are essential for successful fatherhood in cancer patients.
Until recently in Germany, FP services were not covered by health insurances. Only in a few cases, foundations supported individual patients and families. Following the initiative of the foundation for young adults with cancer (Stiftung für junge Erwachsene mit Krebs) and the German Society of Hematology and Cancer (DGHO), a health policy series on FP in patients who receive(d) gonadotoxic treatment was released in November 2017 (Bokemeyer et al. 2017). Political discussions in this context, supported by numerous medical societies, were accompanied by media reports, and eventually resulted in the amendment of the underlying law (Sozialgesetzbuch, SGB V) in 2019, which obliged health insurances to cover FP in patients who receive(d) gonadotoxic treatment. It took until 2021 to finalize interdisciplinary discussions, led by the joint federal committee (Gemeinsamer Bundesausschuss, GBA), to determine service providers and recipients and to finalize the respective billing numbers. Growing demand for cryopreservation among cancer patients, and issues regarding the implementation of the new legal situation require continuous joint efforts to enable all patients entitled to these services to have their costs covered as quickly as possible.
Objective
The present study describes the characteristics of male cancer patients who used the FP service at the Cryobank, Clinic for Urology, Charité-Universitätsmedizin Berlin, Germany between 03/2004 and 05/2019, and of their cryopreserved samples. We additionally assessed utilization rates of these samples for ART and the respective outcomes.
Material and methods
Study population and data collection
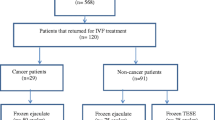
Overall, 1073 sperm and testicular tissue samples from 919 men were cryopreserved at the Cryobank of the Clinic for Urology at the Charité-Universitätsmedizin Berlin, Germany between 03/2004 and 05/2019 (Supplement Fig. 1). Sample and patients medical data were traced from our hospital case notes from 01/2020 to 09/2021, and identified 506 cancer patients for whom oncologic treatment data were available. At the time of study conduction, 46 cancer patients (9.1%) had previously requested their samples for ART. We requested informed consent from these men to additionally obtain fertility cycle information from the respective fertility centers in Germany in which they had chosen to undergo ART, as we do not provide this service in-house. Our study was approved by the ethic committee of Charité-Universitätsmedizin Berlin (EA4/158/19).
Cancer diagnosis and therapy groups
Underlying cancer diagnoses were classified as hematological malignancies, brain tumors, testicular tumors, and non-testicular tumors. Gonadotoxic-risk of previous cancer treatment was defined according to FP guidelines (Dittrich et al. 2018).
Semen and testicular tissue analyses and cryopreservation
Semen analyses were performed before cryopreservation according to the valid WHO laboratory manual of 1999 or 2010 (World Health Organization 1999, 2010) and assessed volume (ml), pH value, sperm concentration (106/ml), motility (a + b + c%), and vitality (%). Men with a low sperm count or a sample volume below WHO reference value were advised to provide additional samples. In case of very low sperm counts/azoospermia, the ejaculate was centrifuged, and the sediment was cryopreserved if motile sperm were present or if patients insisted. Men with azoospermia were advised to cryopreserve testicular tissue. Testicular tissue samples (as big as a grain of rice) were analyzed within 1 h after collection according to the WHO guidelines assessing number of sperms per facial field and motility (%). Samples with no visible sperm under the microscope and a Johnsen Score ˂7 (determined by a pathologist (Johnsen 1970)) were cryopreserved only if patients insisted.
A sample’s banking status was categorized as “ongoing storage”, “transferred to other fertility centres”, “electively discarded”, or “discarded because of patient’s death”.
Assessment of fertility outcome
We assessed maturation, fertilization, pregnancy, miscarriage, and live birth rates following ART with cryopreserved samples. WHO definitions were used to describe perinatal outcomes (gestational age, weight, and height at delivery (World Health Organization 2004).
Statistical methods
Data analysis was conducted using R, version 3.6.1. All continuous variables are presented as means and standard deviations (SD) or median values and interquartile ranges (IQR). Comparisons with numerical variables were made using the one-way ANOVA or Kruskal–Wallis test depending on data distribution. p values < 0.05 were considered statistically significant.
Results
Patient characteristics and description of samples
Out of 506 cancer patients, 53 (10.5%) were younger than 18 years old at cancer diagnosis (mean age total population 29.4 ± 9.1 years). Patient and sample characteristics are presented in Table 1. In total, patients had cryopreserved 601 samples, of which the majority was collected before the initiation of an oncologic treatment (76.7%, 460/600). While both adolescents and adults had mainly banked semen, the rate of testicular tissue cryopreservation was higher in adolescents compared to adults before (11.8%, 6/51 vs. 6.4%, 26/406) and following the start of cancer treatment (16.7%, 4/24 vs. 7.8%, 13/167). Generally, the number of adults who cryopreserved in our center increased over the years (2004–2019), whereas annual numbers of samples provided by adolescents were constantly low (Fig. 1). Semen analyses revealed normospermia in 52.5% (290/552) of patient’s samples. In testicular tissue, spermatozoa were found in 66.7% (28/42) of samples. According to the examination specifications from Johnson et al. 1980, at least 25 tubules of testicle tissue samples were subjected to histometric analysis in the pathology department to assess daily sperm production and thus the degree of maturity of spermiogenesis (Johnsen Score). The Johnsen Score was determined in 15 samples, out of which 7 (46.7%) had a Johnsen Score of 8 or more. At the time of study conduction, storage had already been ended for almost half of all samples collected (44.8%, 269/601). Of these, more than half had been disposed on the patient’s behalf (59.3%, Table 2). A total of 46 patients had previously requested their samples for ART (9.1%), and most (71.5% (28/39) within the first three years after cryopreservation (Supplement, Fig. 2).
Annual numbers of sperm and testicular tissue samples cryopreserved by male adolescent and adult cancer patients between 03/2004 and 05/2019, shown by year of cryopreservation. Overall, 601 samples were stored, of which 554 were sperm (501 adults and 53 adolescents) and 47 testicular tissue samples (37 adults and 10 adolescents)
ART and perinatal outcome
Among the 46 patients who had previously collected a total of 58 samples for ART, only one (2.2%) was an adolescent at time of cancer diagnosis. Mean age at sample collection was 38.0 ± 6.9 years. Patients with non-testicular cancer (17.1%, 18/105) were most likely to collect their samples, followed by patients with hematological malignancies (7.9%, 15/191), testicular tumors (6.3%, 12/192), and brain tumors (5.6%, 1/18). Former testicular cancer patients requested their samples for ART after a shorter period after cryopreservation (19.1 ± 16.0 months, p = 0.521) than those with a non-testicular tumor (25.9 ± 37.9 months) or a hematological malignancy (28.4 ± 40.5 months). Most patients who had requested their samples for ART had cryopreserved these before treatment (71.7%, 33/46). Only 17.24% of samples collected for ART were testicular tissue (17.24%, 10/58).
We received detailed information on 37 fertility cycles conducted in 21 out of the 46 men who underwent ART following collection of their samples stored in our cryobank. None of these men had cryopreserved testicular tissue. Mean age at first ART cycle in these 21 men was 37.7 ± 5.0 years and 33.0 ± 3.5 years in their partners (Table 3). Only two men had used samples that had been cryopreserved following initiation of oncologic treatment (orchiectomy/tumor resection) and 4 men (19.0%) attempted to use fresh sperm which were collected following oncologic treatment for ART. Among men who had used samples cryopreserved before treatment initiation, the majority (76.9%, 10/13) had eventually received a high-gonadotoxic-risk treatment.
Overall, 19 pregnancies in 37 cycles (rate 51.4%) were documented in 16 patients. Only one out of the four men who used fresh sperm achieved a pregnancy by homologous intrauterine insemination (IUI-H). Another patient who initially attempted to use fresh sperm showed severe oligoasthenoteratozoospermia in a first and second cycle but eventually achieved a pregnancy following a third cycle using his previously cryopreserved sperm. Pregnancies resulted in 11 singleton live births, three twin pairs, and four miscarriages (which occurred in two patients). Live-birth rate for the whole population was 65% (13/20) and 76.5% (13/17) for the patients who used cryopreserved samples. Complications were reported for two partners during pregnancy: one had preeclampsia, and the second one threat of premature birth. Further perinatal outcome data are shown in Table 3.
Discussion
We present our monocentric 15-year experience of sperm and testicular tissue cryopreservation in cancer patients. Our study adds information to knowledge on cryopreservation practices in male cancer patients, also addressing adolescent cancer patients, and on outcomes of ART using these cryopreserved samples.
The number of patients who stored samples in our cryobank has grown noticeably from 2004 to 2019. Awareness of cancer treatment-related infertility has increased, particularly in recent years. Resulting in more pronounced attention to FP in clinical standards, and to the introduction of guidelines for childhood, adolescent, and adult cancer patients (Dittrich et al. 2018; Lambertini et al. 2020). The increase of patients undergoing cryopreservation in our cryobank may be attributed to promoting developments of the department itself and/or cooperating institutes. This includes expanding patient education with the help of advertisement and brochures, websites or lectures, improved supply, and growing cooperation with clinics/private practices in the federal states of Berlin and Brandenburg. We collected retrospective cryopreservation data until 05/2019. A few months later, the legal basis obliging health insurance coverage of FP was achieved in Germany. Since then, an even stronger annual rise in the number of cryopreserved samples in our department is noticeable (2020: n = 110, 2021: n = 128, not shown in results). Reports from other countries in which a public funding program has been implemented similarly show increasing cryopreservation rates (Herrero et al. 2016). Yet, funding of cryopreservation is not implemented across all European countries and not all patients have equal access to FP (European atlas of fertility treatment policies 2021). Networks such as the interdisciplinary paneuropean late effect network, PanCare, advocate to improve equal chances in treatment for patients throughout Europe (Mulder et al. 2021). In Germany, Austria, and Switzerland, e.g., the FertiProtekt network greatly contributes to improved accessibility of FP for cancer patients (FertiPROTEKT Netzwerk 2006).
Recent studies reveal generally low cryopreservation rates among cancer patients (Bizet et al. 2012; Mulder et al. 2021), e.g., in a current study less than half of adolescent cancer patients cryopreserved samples (Balcerek et al. 2020). Barriers in utilization include financial issues, availability of measures, insufficient time for FP due to urgency of cancer treatment, patient’s chance of survival, insufficient advice, cultural and religious beliefs, a previous fatherhood status, or indecision about wanting to be a parent (Halpern et al. 2020). In our study, most cryopreserved samples (75.6%) were collected by either patients with a testicular cancer or a hematological malignancy. These results are similar to previous studies with numbers of utilization ranging from 19 to 41.7% for testicular cancer and 35.4 to 71.7% for hematological malignancies (Bizet et al. 2012; Depalo et al. 2016; Reschini et al. 2021). Among all samples cryopreserved in our department, only 10% were collected from under-aged cancer patients. However, future parenthood is already a topic of relevance in adolescents (Picton et al. 2015). FP poses a particular challenge in children and adolescents with cancer (Picton et al. 2015). While adolescents are being offered long-established sperm and/or testicular tissue cryopreservation in our cryobank, FP in prepubertal patients is only available in experimental settings (Kabiri et al. 2022). Immature testicular tissue cryopreservation in prepubertal boys is a promising technique (Kabiri et al. 2022). We offer this procedure in collaboration with the Androprotect Study (Universitätsklinik Münster, Germany). Despite advances of reproductive medicine, further knowledge on the specific risks of cancer treatment is still required to improve individual counseling and FP strategies for cancer patients.
Due to impaired fertility following cancer treatment, a rising number of cancer survivors use ART to fulfill their desire for a child of their own (Verona et al. 2021). In Germany, almost twice as many survivors reported having conceived following ART than rates published for the general population (2.6 vs. 4.6%) (Sommerhäuser et al. 2021). In our study, only 9.1% of cryopreserved samples were eventually used for ART, which is comparable with the aggregated usage rate of 8% published by Ferrari and colleagues who reviewed 30 studies (Ferrari et al. 2016). Some of the reasons for survivors for not having used their cryopreserved samples included having achieved conceptions naturally, no desire to have a child (yet) or follow-up period after cryopreservation being too short for especially adolescents. In our study, most samples that were requested for ART had been cryopreserved before a high-gonadotoxic-risk cancer treatment. The majority of patients had used their samples within the first 3 years following cryopreservation, with utilization rates further decreasing from 4 to 12 years since cryopreservation. Similar trends were reported previously (Depalo et al. 2016), with none of the cryopreserved samples having been used after a follow-up period of 15 years (Kelleher et al. 2001). Patients in our study collected their samples at a mean age of 38.00 ± 6.87 years, which may be related to a generally higher age of first fatherhood in European countries (average 34.6 years) (Federal Statistical Office of Germany 2020) and to the observation that cancer patients tend to reach milestones later in life compared to peers (Langeveld et al. 2003). Only one adolescent cancer survivor had collected his samples for ART. However, it needs to be noted that mean age at diagnosis of adolescent cancer patients in our cohort was 16 ± 1.5 years, and at study time 21 ± 4.2 years, suggesting that follow-up time was too short to examine utilization rate for these patients. Although freezing and thawing procedures potentially decrease sperm motility and total motile sperm count (Kelleher et al. 2001), most patients in our cohort successfully achieved pregnancies using their cryopreserved sperm (56.3%), of which 76.5% resulted in live births. These results are similar to other studies (Fu et al. 2019), which reported a pregnancy rate of 51.5% (17/33) and a live birth rate of 71.4% (10/14) following ART with cryopreserved samples. Half of our patients and their partners only required one ART cycle to achieve a pregnancy, and none required more than three cycles, which is reassuring. In our cohort, in line with results of other studies (Depalo et al. 2016), the majority of patients underwent ICSI (75.7%), which is more efficient in case of severe male factor infertility compared to IVF (Haddad et al. 2021), and reduces the risk of failed fertilization (Depalo et al. 2016). In our population, only one pregnancy was reported after the use of fresh sperm. Adverse perinatal outcomes occurred in a fifth to a quarter of pregnancies, such as prematurity (23.1%), low birth weight (21.4%), and/or cesarean section (25.0%), which can be associated with the higher prevalence of multiple sibling births following ART compared to following natural conception (Sommerhäuser et al. 2021). In our cohort, 21.4% of births following ART were multiple sibling births. Nowadays, fewer embryos are used per transfer than at the beginning of the observation period, and consequently, the multiple-birth rate has been reduced (Verona et al. 2021). ART in cancer patients has not been associated with increased risk of congenital abnormalities or adverse health outcomes (Picton et al. 2015).
Study limitations
Limitations regarding our study design need to be taken into account. Due to the retrospective setting, we were only able to collect information on those patients who cryopreserved their samples in our cryobank. However, no information was available on patients who ultimately did not store samples; similarly, we cannot provide reasons for why these samples were not stored.
Moreover, it should be noted that the clinical practice of FP recommendations and oncological treatment strategies have changed over the years of the retrospective study period. Unfortunately, we saw, that despite these changes, numbers of cryopreservation in adolescents remained low over the period of 15 years. Specifically adolescents would, however, benefit from FP and enhance their chances of a future parenthood. In accordance to guideline recommendations, the majority of samples were cryopreserved before start of the treatment. Therefore only little information is available on samples collected after treatment. Information on outcome using these samples is relevant for counseling patients who are unable to cryopreserve before initiation of a gonadotoxic treatment. No follow-up on natural conceptions was documented for patients. As we do not provide in-house ART, we could only address those patients to provide information on ART cycles who had previously requested their samples from our department. ART was conducted in external fertility centers chosen by the patients, resulting in variances of ART procedures between centers. Future studies should examine the success of ART using testicular tissue samples, which was not possible in the current study as none of the patients had requested their testicular tissue for ART.
Conclusions
We present our monocentric experience of FP in male cancer patients, including adolescents. As recommended in guidelines, the majority of patients had cryopreserved sperm samples and/or testicular tissue samples prior to cancer treatment. Cryopreservation rates among adolescents were low with little increase over time. Overall, only 10% of samples cryopreserved were used for ART. Existing fears of using cryopreserved samples for ART, and success rates, should be discussed in detail with patients. Our results of outcomes following ART with cryopreserved samples are reassuring regarding the efficiency and perinatal outcomes. However, outcomes need to be confirmed in a larger cohort. With the new advantage of public health insurance coverage of FP in Germany, the number of those turning to cryopreservation in the context of potential gonadotoxic treatment has already risen, emphasizing the necessity of further knowledge for individual counseling.
Data sharing statement
The data underlying this article will be shared on reasonable request to the corresponding author.
References
Balcerek M, Schilling R, Byrne J, Dirksen U, Cario H, Fernandez-Gonzalez MJ, Kepak T, Korte E, Kruseova J, Kunstreich M, Lackner H (2020) Determinants of utilization of cryopreservation of germ cells in adolescent cancer patients in four European Countries. Eur J Pediatr 179(1):51–60. https://doi.org/10.1007/S00431-019-03459-9
Bizet P, Saias-Magnan J, Jouve E, Grillo JM, Karsenty G, Metzler-Guillemain C, Perrin J (2012) Sperm cryopreservation before cancer treatment: a 15-year monocentric experience. Reprod Biomed Online 24(3):321–330. https://doi.org/10.1016/J.RBMO.2011.11.015
Bokemeyer B-S, Dittrich, Freund OM (2017) Vom Krebs Geheilt, Aber Nicht Gesund Keine Hoffnung Auf Eigene Kinder Band 11 In Zusammenarbeit Mit: Deutsche Stiftung Für Junge Erwachsene Mit Krebs.
Chong AL, Gupta A, Punnett A, Nathan PC (2010) A cross canada survey of sperm banking practices in pediatric oncology centers. Pediatr Blood Cancer 55(7):1356–1361. https://doi.org/10.1002/PBC.22705
Depalo R, Falagario D, Masciandaro P, Nardelli C, Vacca MP, Capuano P, Specchia G, Battaglia M (2016) Fertility preservation in males with cancer: 16-year monocentric experience of sperm banking and post-thaw reproductive outcomes. Ther Adv Med Oncol 8(6):412–420. https://doi.org/10.1177/1758834016665078
Dittrich K, Schüring B, Baston-Büst B, Beckmann B, Borgmann-Staudt C, Denzer D, Dorn F, Gaase G, Geue G, Goeckenjan G, Guth H, Hehr H, Hirchenhain H, Hornemann J, Kentenich K, Köhn K, Lax L, Lux M, Micke N, Nawroth N, Ochsendorf O, Pelz R, Reisch R, Schlatt S, Schwab S, Thorn W, Wildt W, Wischmann VW, Lotz A (2018) Fertility preservation for patients with malignant disease. Guideline of the DGGG, DGU and DGRM (S2k-Level, AWMF Registry No. 015/082, November 2017). Recommendations and statements for girls and women. Geburtshilfe Frauenheilkd 78(6):567–584. https://doi.org/10.1055/A-0611-5549
European Atlas of Fertility Treatment Policies-Fertility Europe (2021). https://fertilityeurope.eu/european-atlas-of-fertility-treatment-policies/.
Federal Statistical Office of Germany (2020). https://www.destatis.de/EN/Home/_node.html
Ferrari S, Paffoni A, Filippi F, Busnelli A, Vegetti W, Somigliana E (2016) Sperm cryopreservation and reproductive outcome in male cancer patients: a systematic review. Reprod BioMed 33(1):29–38. https://doi.org/10.1016/J.RBMO.2016.04.002
FertiPROTEKT Netzwerk e.V. (2006). https://fertiprotekt.com/en/
Fu L, Zhou F, An Q, Zhang K, Wang X, Xu J, Guo Y, Lu W, Liang X, Gu Y (2019) Sperm cryopreservation for male cancer patients: more than 10 years of experience, in Beijing China. Med Sci Monit 25:3256. https://doi.org/10.12659/MSM.913513
Haddad M, Stewart J, Xie P, Cheung S, Trout A, Keating D, Parrella A, Lawrence S, Rosenwaks Z, Palermo GD (2021) Thoughts on the popularity of ICSI. J Assist Reprod Genet 38(1):101–123. https://doi.org/10.1007/S10815-020-01987-0/FIGURES/3
Halpern JA, Das A, Faw CA, Brannigan RE (2020) Oncofertility in adult and pediatric populations: options and barriers. Transl Androl Urol 9(2):S227. https://doi.org/10.21037/TAU.2019.09.27
Herrero G, Buckett T, Chan P (2016) Quebec public funding facilitates fertility preservation for male cancer patients. Curr Oncol 23(1):20–25. https://doi.org/10.3747/co.23.2793
Johnsen SG (1970) Testicular biopsy score count – a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Horm Res Paediatr 1(1):2–25. https://doi.org/10.1159/000178170
Kabiri S, Gropp H, Mordechai-Daniel M, Revel I, Reubinoff. B (2022) Establishment of a controlled slow freezing-based approach for experimental clinical cryopreservation of human prepubertal testicular tissues. F&S Rep 3(1):47–56. https://doi.org/10.1016/J.XFRE.2021.11.001
Kelleher S, Wishart SM, Liu PY, Turner L, Di Pierro I, Conway AJ, Handelsman DJ (2001) Long-term outcomes of elective human sperm cryostorage. Hum Reprod 16(12):2632–2639. https://doi.org/10.1093/humrep/16.12.2632
Korte E, Schilling R, Balcerek M, Byrne J, Dirksen U, Herrmann G, Kepak T, Klco-Brosius S, Kruseova J, Kunstreich M, Langer T (2020) Fertility-related wishes and concerns of adolescent cancer patients and their parents. J Adolesc Young Adult Oncol 9(1):55–62. https://doi.org/10.1089/JAYAO.2019.0064
Lambertini M, Peccatori FA, Demeestere I, Amant F, Wyns C, Stukenborg JB, Paluch-Shimon S, Halaska MJ, Uzan C, Meissner J, Von Wolff M (2020) Fertility preservation and post-treatment pregnancies in post-pubertal cancer patients: ESMO clinical practice guidelines. Ann Oncol. https://doi.org/10.1016/j.annonc.2020.09.006
Langeveld U, Last G, De Voute H (2003) Educational achievement, employment and living situation in long-term young adult survivors of childhood cancer in the Netherlands. Psychooncology 12(3):213–225
Maroufizadeh O-S, Bagheri-Lankarani A-H, Amini P (2018) Factors associated with poor well-being of infertile people: a cross-sectional study. Middle East Fertility Society Journal 23(4):468–470. https://doi.org/10.1016/J.MEFS.2018.03.006
Mulder RL, Font-Gonzalez A, Green DM, Loeffen EA, Hudson MM, Loonen J, Yu R, Ginsberg JP, Mitchell RT, Byrne J, Skinner R (2021) Fertility preservation for male patients with childhood, adolescent, and young adult cancer: recommendations from the pancarelife consortium and the international late effects of childhood cancer guideline harmonization group. Lancet Oncol 22(2):e57-67. https://doi.org/10.1016/S1470-2045(20)30582-9
Okada K, Fujisawa M (2019) Recovery of spermatogenesis following cancer treatment with cytotoxic chemotherapy and radiotherapy. World J Men’s Health 37(2):166–174. https://doi.org/10.5534/WJMH.180043
Papler TB, Vrtacnik-Bokal E, Drobnic S, Stimpfel M (2021) The outcome of IVF/ICSI cycles in male cancer patients: retrospective analysis of procedures from 2004 to 2018. Radiol Oncol 55(2):221–228. https://doi.org/10.2478/RAON-2021-0011
Picton D, Wyns D, Anderson D, Goossens D, Jahnukainen D, Kliesch D, Mitchell D, Pennings D, Rives D, Tournaye D, van Pelt D, Eichenlaub-Ritter D, Schlatt S (2015) A European perspective on testicular tissue cryopreservation for fertility preservation in prepubertal and adolescent boys. Hum Reprod 30(11):2463–2475. https://doi.org/10.1093/HUMREP/DEV190
Reschini S, Meazza P, Guarneri G, Massimino R, Filippi F, Terenziani. M (2021) Sperm cryopreservation in adolescents with cancer. Eur J Obstetr Gynecol Reprod Biol 260:198–202. https://doi.org/10.1016/J.EJOGRB.2021.03.041
Schover B, Lichtin L, Jeha. S (2002) Knowledge and experience regarding cancer, infertility, and sperm banking in younger male survivors. J Clin Oncol 20(7):1880–1889. https://doi.org/10.1200/JCO.2002.07.175
Sommerhäuser B-S, Astrahantseff B, Calaminus D, Fernández-González H, König S, Schuster L, Balcerek. M (2021) Health outcomes in offspring born to survivors of childhood cancers following assisted reproductive technologies. J Cancer Surviv 15(2):259–272. https://doi.org/10.1007/S11764-020-00929-0/TABLES/4
Verona B, Czeromin U, Daniel F, Klaus F, Gnoth C (2021) German IVF Registry (D.I.R) annual report 2021. J Fur Reproduktionsmedizin Und Endokrinol 15:217–250. https://doi.org/10.1038/news051017-6
World Health Organisation (2004) International statistical classification of diseases and related health problems (ICD). Alphabetical index, edn 3. World Health Organization, Geneva
World Health Organization (1999) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, Cambridge (Published on behalf of the World Health Organization)
World Health Organization (2010) WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge University Press, Cambridge (Published on behalf of the World Health Organization)
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors would like to thank Stiftung für Urologische Forschung for funding of our study (01/2020-01/2021).
Author information
Authors and Affiliations
Contributions
MJF-G, AB-S, MB, and IW contributed to the study conception and design. MB and IW acquired funding. Material preparation was performed by MJF-G and MB. Data collection were performed by MJF-G, ACCRP, MB, and IW. Data validation and formal analysis was conducted by MJFG. The manuscript draft was written by MJFG and MB, with editing by ACRP, ABS, and IW. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
The study was approved by the ethical board of Charité-Universitätsmedizin Berlin (EA4/158/19).
Informed consent
Informed written consent was obtained from all patients prior to their enrollment in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fernández-González, M.J., Radauer-Plank, AC., Stelzer, C. et al. Sperm and testicular tissue cryopreservation and assisted reproductive technology outcomes in male cancer patients: a 15-year experience. J Cancer Res Clin Oncol 149, 5321–5330 (2023). https://doi.org/10.1007/s00432-022-04488-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04488-y